Alkenes Part 1.
Hello, friends of steemit, I want to share with you some basic, but important information about alkenes. This is the first part of the topic. Where we will go into its synthesis.
Synthesis of alkenes
- Dehydration of alcohols:
This method makes use of a strong inorganic acid such as concentrated sulfuric acid as a catalyst. The alcohol is heated to reflux in this mixture and the alkene product is subsequently distilled.
The reaction proceeds in several stages, with the formation of a carbocation as an intermediate in the slow stage.
The speed of the reaction will depend on the stability of the carbocation that forms.
The more stable it is, the quicker the dehydration reaction will be. The great disadvantage of this method lies in the possibility of rearrangement of the carbon skeleton.
- Dehydrogenation of alkyl halides:
This reaction occurs in the presence of a strong base such as potassium hydroxide in ethanol or other bases such as sodium ethoxide in ethanol or alkoxide in their respective alcohol. The use of small bases (KOH-Ethanol) produces more substituted alkenes, whereas the use of bulky bases such as terbutanol sodium tert-butoxide preferably produces another alkene, different from the more substituted one.
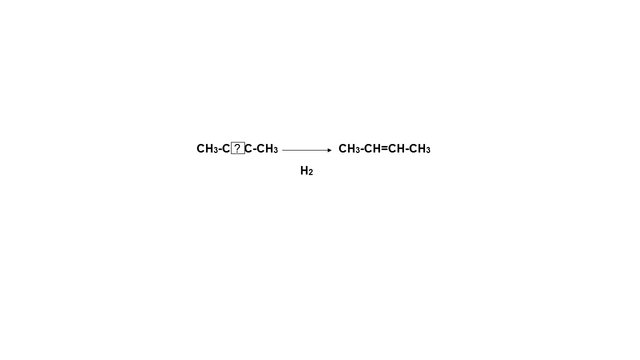
- Hydrogenation of alkynes:
The hydrogenation is an exothermic reaction, and the heat released is affected by the alkyne substituents. Thus, the internal alkynes give off less heat when they are hydrogenated than the terminals, due to their greater stability due to the phenomenon of hyperconjugation.
For more information, you can visit the following links:
- https://crab.rutgers.edu/~alroche/Ch07.pdf
- https://www.universalclass.com/articles/science/organic-chemistry/properties-synthesis-and-reactions-of-alkenes-and-alkynes.htm
- https://chem.libretexts.org/Core/Organic_Chemistry/Alkenes/Synthesis_of_Alkenes
![1.1- ejemplo 1 sintesis de alquenos[513].jpg](https://steemitimages.com/640x0/https://steemitimages.com/DQmeQuwPxSphNNkV47EBXMZWmny5jbghbqCzD1aDBnQb2Vx/1.1-%20ejemplo%201%20sintesis%20de%20alquenos%5B513%5D.jpg)
![1.2- ejemplo 2 sintesis de alquenos[514].jpg](https://steemitimages.com/DQmNuQ97NhsVrJZyMiRMrWc2r3gDABR87KwhtNgL1wBQKro/1.2-%20ejemplo%202%20sintesis%20de%20alquenos%5B514%5D.jpg)
Very educational, I really liked your post.