Water electrolysis: the simplest way to obtain the chemical elements of water in a pure way
Water electrolysis: the simplest way to obtain the chemical elements of water in a pure way.
Before going into the subject of water electrolysis, it is necessary to be clear that electrolysis is an electrochemical oxidation-reduction process that takes place when electrical energy is passed through a molten electrolyte or aqueous solution.
Water Electrolysis.
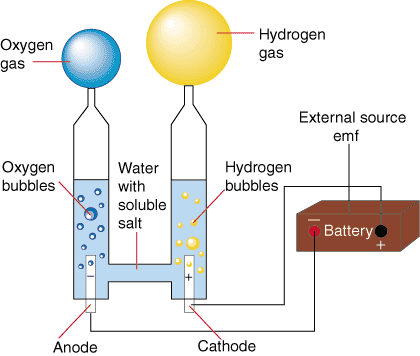
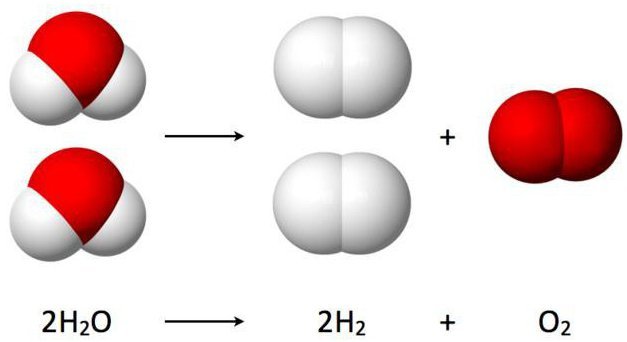
The electrolysis of water basically consists of a process through which water is divided into Hydrogen and Oxygen. By subjecting the substance to the current both electrodes will present gas evolution, the hydrogen gas will be released through the cathode, while the oxygen will be released from the anode.
Source
For this process to happen, the following conditions must be met:
• Water cannot be in a pure state, that is, it must have small concentrations of salts or other minerals.
• A direct current must be used in this process.
Process.
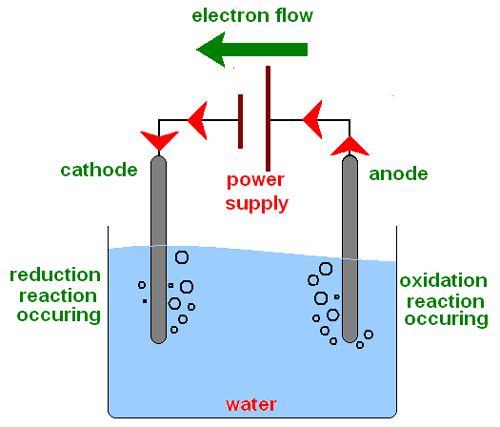
In this process, the cathode is called the electrode that contributes the negative charge to the aqueous solution or molten electrolyte, while the other electrode that is responsible for the negative charge is called the anode.
The electrolysis of water allows obtaining the chemical elements that compose it in a pure way that is hydrogen (H) through the cathode and oxygen (O) through the anode, both in a gaseous state.
This process can be applied to fresh and salt water, obtaining different results taking into account the product substances. In the case of salt water, the substances obtained through the anode and the cathodes are chlorine (Cl) and hydrogen (H) respectively both in the gaseous state, leaving at the end of the process a solution of sodium hydroxide (NaOH).
Source
Applications.
The electrolysis of the brine (salt water), a fairly concentrated mixture of sodium chloride and water, is only half the electrolysis of water since the chloride ions in the brine solution are oxidized to chlorine instead to oxidize water to oxygen due to its normal electrode potential. The hydrogen produced from this process is burned (converted back into the water), and used for the production of chemical specialties, or several other small-scale applications.
As a specific application it is interesting to mention that the electrolysis of water is also used to generate oxygen in the International Space Station which obviously serves to maintain the atmosphere of the station and hydrogen can be used later in a fuel cell as a method to store energy and use the water that is generated in the cell for consumption by astronauts.
Source
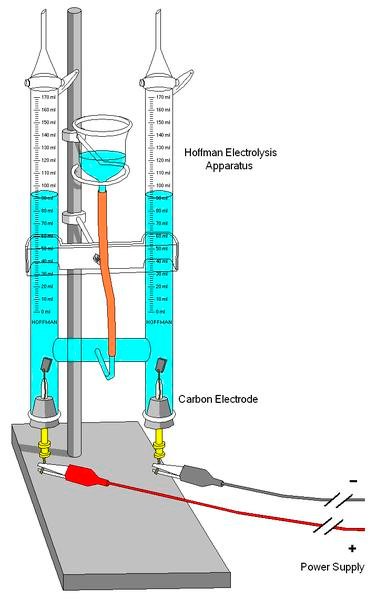
Hoffman Voltmeter.
The use of Hoffman's voltmeter allows the electrolysis of water in a very comfortable way by collecting hydrogen and oxygen gases in each of the tubes. In this way, the visitor observes that the volume of hydrogen that is produced is double that of oxygen; demonstrating what is the chemical composition of water. The energy needed to separate the ions of the molecule from the water is provided by the power source. This is, therefore, an endothermic reaction.

Source
The water must contain a small amount of sulfuric acid to improve its conductivity. In the tube connected to the electrode of the positive pole (cathode), oxygen is collected. To check it, you can zoom in on the ember of a flat toothpick. It will be shown how oxygen fans the flame and sometimes even turns on again.
Source
Hydrogen is collected in the tube connected to the electrode of the negative pole (anode). To check it, you can do the reverse reaction to the electrolysis of water: the synthesis of water. The tap on the tube containing the hydrogen is opened and collected with an inverted test tube. A spark of a hand-held lighter (activation energy) approaches and the hydrogen reacts with the oxygen in the air producing a peculiar burst.
Source
References:
- http://www.scielo.br/scielo.php?script=sci_arttext&pid=S0100-40422013000800017
- http://www1.lsbu.ac.uk/water/electrolysis.html
- http://www.instructables.com/id/Separate-Hydrogen-and-Oxygen-from-Water-Through-El/
- http://www.molecularhydrogenfoundation.org/electrolysis-2/
- https://en.wikipedia.org/wiki/Electrolysis_of_water#Techniques

This is a test comment, notify @kryzsec on discord if there are any errors please.
Being A SteemStem Member
Picture sources should lead to the page where the picture is embedded, not the picture itself. More specifically to a page that states the license on the pictures.
Sorry, you're Right
No worries, don't feel bad, just for next time.
Thanks for the advice, I really appreciate it. I have already edited the sources correctly.
Congratulations @ideas-abstractas! You have completed some achievement on Steemit and have been rewarded with new badge(s) :
Click on any badge to view your own Board of Honor on SteemitBoard.
For more information about SteemitBoard, click here
If you no longer want to receive notifications, reply to this comment with the word
STOPSTOP
Congratulations! This post has been upvoted from the communal account, @minnowsupport, by Ideas-abstractas from the Minnow Support Project. It's a witness project run by aggroed, ausbitbank, teamsteem, theprophet0, someguy123, neoxian, followbtcnews, and netuoso. The goal is to help Steemit grow by supporting Minnows. Please find us at the Peace, Abundance, and Liberty Network (PALnet) Discord Channel. It's a completely public and open space to all members of the Steemit community who voluntarily choose to be there.
If you would like to delegate to the Minnow Support Project you can do so by clicking on the following links: 50SP, 100SP, 250SP, 500SP, 1000SP, 5000SP.
Be sure to leave at least 50SP undelegated on your account.