Photorespiration - Metabolic Engineering
In a previous post in this series (Photorespiration - Prelude), I calculated that C3 plants lose about 23% of their carbon due to photorespiration when grown at 25oC at sea level. Could this be an erroneous output of my computations? I doubt this because others have reported losses of a similar magnitude. For example Walker et al. (2016) report that photorespiration decreases US soybean and wheat yields by 36% and 20%, respectively. They further note that "a 5% decrease in the losses due to photorespiration would be worth approximately $500 million annually in the United States". Peterhansel and Maurino (2011) also estimate about 25% of the carbon gained by C3 plants in photosynthesis is lost during photorespiration under normal growth conditions, and these losses only increase when C3 plants are subjected to high temperature stress and water deficits.
With these massive losses in crop productivity and potential for huge gains in yield (and crop value) it is no wonder that substantial research effort has been devoted to trying to engineer C3 plants so that photorespiration is significantly reduced.
The strategy for metabolic engineering of this pathway is to insert genes encoding enzymes that bypass the normal photorespiratory nitrogen/carbon cycle. Shown in the image below is the photorespiratory pathway that I previously illustrated in the article Photorespiration - Biochemistry but now drawn with an additional bold arrow (in purple) connecting 2 x glycolate --> glycerate + CO2 in the chloroplast:
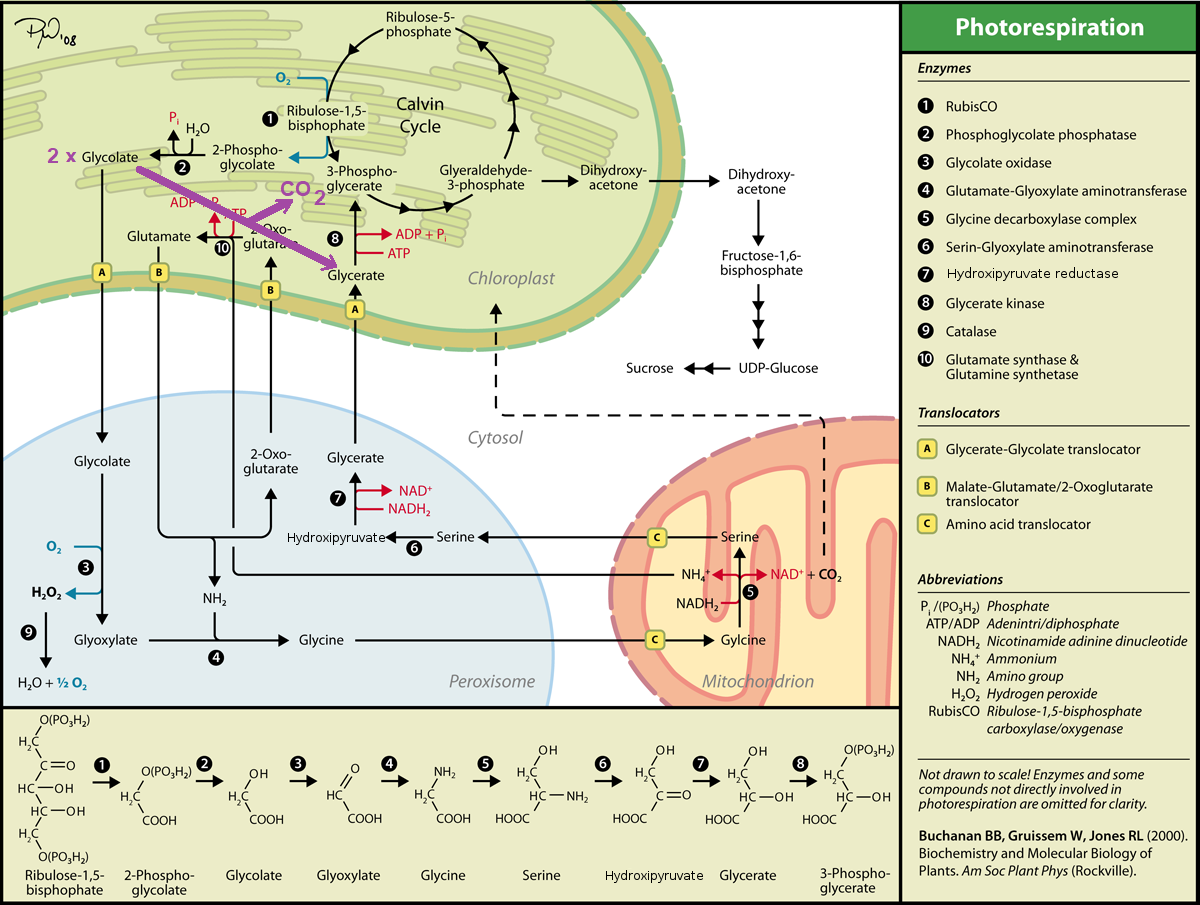
Photorespiration in C3-plants. How plants cope with the oxygenase reaction of RuBisCO (with a bypass drawn in purple). Image Source: Photorespiration - Wikipedia
Such a bypass would prevent the need for exporting glycolate from the chloroplast to the peroxisome, would prevent generation of hydrogen peroxide in the peroxisome, would eliminate the need for synthesis of glycine and serine, would prevent ammonium production in the mitochondrion and prevent energy losses associated with re-assimilation of that ammonium. Furthermore, although this bypass still generates CO2, it does so in the chloroplast, rather than the mitochondrion, making it more readily available for re-assimilation by RuBisCO in the Calvin cycle. One could argue also that with a such a bypass, the elevated level of CO2 in the chloroplast would tend to suppress further the oxygenase activity of RuBisCO responsible for initiating photorespiration.
Such a pathway has indeed been installed in the chloroplasts of the experimental C3 plant Arabidopsis thaliana by Kebeish et al. (2007):
2 x glycolate --GDH--> glyoxylate --GCL--> CO2 + tartronic semialdehyde --TSR--> glycerate
catalyzed by three enzymes glycolate dehydrogenase (GDH), glyoxylate carboxyligase (carboligase) (GCL), and tartronic semialdehyde reductase (TSR). All genes encoding these enzymes were derived from the bacterium E. coli.
Engineered plants of Arabidopis expressing these 3 foreign proteins in the chloroplast showed substantial greater biomass production (by up to 30%) in normal air (Kebeish et al. (2007), Betti et al. (2016)). These same genes were subsequently expressed in the C3 biofuel crop, Camelina sativa, and the engineered plants remarkably showed increased vegetative growth, accelerated development, earlier flowering and seed set, and a 57-73% increase in seed yield with no loss of seed quality (Dalal et al. (2015)).
Other bypasses have also been designed and tested (see review by Peterhansel et al. (2013)), but none seem to be as successful as the bypass described above. One approach attempted by Carvalho et al. (2011) was to insert genes that create a bypass between glyoxylate and hydroxypyruvate in the peroxisome, generating CO2 in the peroxisome of tobacco plants using genes (from E. coli) encoding glyoxylate carboligase (GCL) and hydroxypyruvate isomerase (HYI). While GCL was successfully expressed, no expression of HYI was observed and the resulting plants did not perform as well as non-engineered controls in normal air. The incomplete bypass installed seemed to create a deleterious short-circuit of the photorespiratory nitrogen cycle (Carvalho et al. (2011)).
Yet another approach has been to convert 2 x glycolate into 2 x glyoxylate in the chloroplast using a chloroplastic glycolate oxidase, convert the resulting glyoxylate to malate (generating CO2 in the chloroplast), and then decarboxylate the malate to pyruvate and CO2 in the chloroplast of Arabidopsis. While this particular bypass does not regenerate glycerate it results in greater CO2 levels in the chloroplast, which may help suppress the oxygenase activity of RuBisCO. Although this particular bypass may have higher energy costs than those described above, it successfully enhanced biomass production by 30% (for more details of this pathway, please see Betti et al. (2016) and Peterhansel and Maurino (2011)).
Given the dramatic effects of 2 out of 3 of these engineered bypasses on the productivity of C3 plants and yield, there is a great deal of interest in pursuing these strategies to provide food for a growing world population. However, further research is still needed to determine whether any adverse effects are associated with these bypasses under field conditions. How will these bypasses influence glycine and serine synthesis and their connections to central carbon and nitrogen metabolism? Will these bypasses influence plant stress tolerance and/or resistance to pathogens and insect attack? What will be their impact on nitrogen nutrition and the phenonomenon of photoinhibition (discussed in my previous article on Photosynthesis - The Light Reactions)? Whether such genetically engineered (GMO) plants will be accepted by the world population is also another extremely important question that is beyond the scope of the present article.
I encourage readers to consult the literature cited below and to form their own opinion about these engineering strategies, their potential impacts on crop productivity, possible (unanticipated) pitfalls that may be encountered, and whether these strategies will be embraced or rejected by human kind. Please feel free to express your own opinions on these matters in the comments section below.
References
Peterhansel, C., Maurino, V.G. Photorespiration redesigned. Plant Physiol. 155: 49-55 (2011)