Floral Scent - Part 3
This post is a continuation of my earlier posts: Floral Scent - Part 1, and Floral Scent - Part 2. Here I will focus on snapdragon flowers. Unlike (Petunia hybrida), snapdragon (Antirrhinum majus, a member of the family Scrophulariaceae) is pollinated by bumblebees, and emits floral scent primarily during the day (coinciding with pollinator activity) rather than at night.

Image From: Understanding of floral scents blossoms in Purdue laboratory (Purdue News (2000)).
While Petunia hybrida flowers emit only volatile benzenoids/phenylpropanoids, snapdragon flowers emit a complex blend of benzenoids/phenylpropanoids and terpenoids/isoprenoids (both monoterpenes and sesquiterpenes) (Weiss et al. (2016)). But there is substantial variability among Antirrhinum species with respect to the composition of the floral bouquets. Some Antirrhinum species also emit fatty acid derivatives and a nitrogen containing compound, indole:
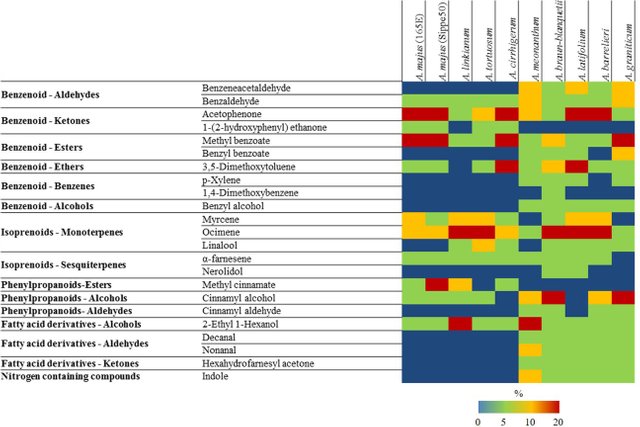
Heat map with major volatiles emitted by different species of Antirrhinum. Image from: Weiss et al. (2016).
Methyl benzoate is one the major floral scent components of the benzenoid class in Antirrhinum majus and the synthesis of this compound is catalyzed by S-adenosyl-L-methionine:benzoic acid carboxyl methyltransferase (BAMT), an enzyme that adds a methyl group to the carboxyl moiety of benzoic acid. The expression of this enzyme is moderately diurnally regulated, but the primary factor determining synthesis of methyl benzoate appears to be the supply of benzoic acid rather than the level of the BAMT in snapdragon (Kolosova et al. (2001)).
Biosynthesis of benzoic acid in Antirrhinum majus appears to occur predominantly via the catalytic action of an enzyme that converts benzaldehyde to benzoic acid, benzaldehyde dehydrogenase (BALDH) (Long et al. (2009)).
Because I have discussed the benzenoid/phenylpropanoid biosynthetic pathways at length in previous posts in this series, I will not reiterate these pathways here. Suffice it to say that the amino acid phenylalanine provides the carbon skeletons for these benzenoid/phenylpropanoid molecules. Rather, I would like to focus on the terpenoids/isoprenoids produced by Antirrhinum majus. Here it gets somewhat complicated. There are two alternative routes of synthesis of terpenoids/isoprenoids in plants; a cytosolic pathway (termed the mevalonic acid (MVA) pathway), and a plastid/chloroplastic pathway (termed the methylerythritol phosphate (MEP) pathway). The cytosolic pathway generates sesquiterpenes (with 15 carbon atoms), while the plastidic pathway generates monoterpenes (with 10 carbon atoms). These pathways also produce a number of other important plant chemicals including hormones (brassinosteroids, abscisic acid (ABA)) and pigments (chlorophylls and carotenoids). There may be some "cross-talk" between pathways via exchange of a common intermediate, isopentenyl diphosphate (IPP), between cytosol and plastid, as indicated with a question mark (?) in the image below:
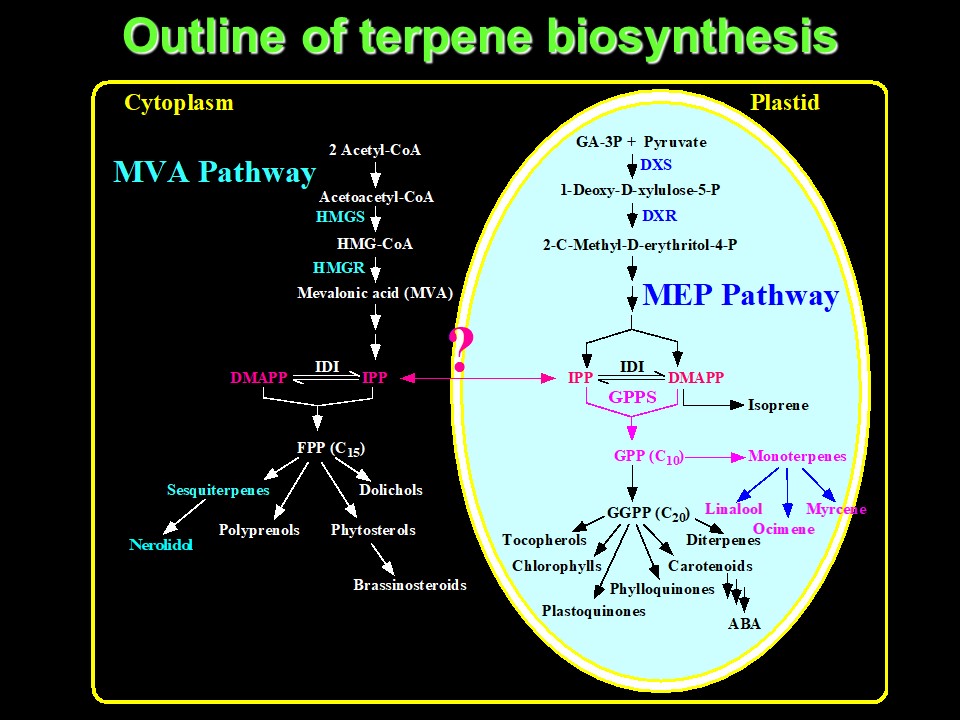
Outline of terpene biosynthesis. Image courtesy of Natalia Dudareva (Purdue University).
The early steps in the cytosolic pathway also occur in humans and are involved in cholesterol biosynthesis. The cholesterol-lowering drugs, statins, are inhibitors of the enzyme 3-hydroxy-3-methylglutaryl-CoA reductase (HMGR)!
Mevalonin also inhibits HMGR and hence the cytosolic pathway, while fosmidomycin is a specific inhibitor of the MEP pathway in the plastid (fosmidomycin specifically inhibits the enzyme 1-deoxy-D-xylulose-5-phosphate reductoisomerase (DXR) in the MEP pathway). Thus, mevalonin and fosmidomycin have found utility in probing the relative contributions of these two pathways to terpenoid/isoprenoid biosynthesis by snapdragon flowers and to address the unresolved question of metabolite exchange between the two pathways.
Terpenoids emitted from snapdragon flowers include three monoterpenes, myrcene, (E)-B-ocimene, and linalool, derived from geranyl diphosphate (GPP), and a sesquiterpene, nerolidol, derived from farnesyl diphosphate (FPP) (Dudareva et al. (2005)). Feeding of snapdragon flowers with pathway-specific precursors (deuterium-labeled 1-deoxy-D-xylulose or deuterium-labeled mevalolactone), with and without the afore-mentioned inhibitors, and monitoring the labeling patterns of terpenoids in floral scent by gas chromatograpy-mass spectrometry (GC-MS), led to the following major conclusions (Dudareva et al. (2005)):
Only one of the two pathways, the plastid-localized MEP pathway, is active in the formation of volatile terpenes.
The MEP pathway provides IPP precursors for both plastidial monoterpene and cytosolic sesquiterpene biosynthesis in the epidermis of snapdragon petals.
The trafficking of IPP occurs unidirectionally from the plastids to cytosol.
The MEP pathway operates in a rhythmic manner controlled by the circadian clock, which determines the rhythmicity of terpenoid emission.
Subsequent studies have led to the identification of two nearly identical terpene synthases that catalyze the formation of nerolidol and linalool in snapdragon flowers (Nagegowda et al. (2008)).
Please feel free to comment or ask questions about this post in the comments section below. I will try to answer questions as soon as possible.
Being A SteemStem Member
Thank you!