Chemistry of Life: Carbon and Mechanism behind our being Alive
Carbon, Skeleton behind life
My last post was on Carbon Monoxide and Carbon Monoxide poisoning. Today, I decided to go deeper into the world of chemical science to talk about an element called Carbon and bring it in relation to biological science. Carbon is a major constituent of the dangerous gas Carbon Monoxide. Carbon being a constituent of the hazardous carbon monoxide gas isn't bad in itself, it is even a major constituent of the food we eat, the air we breath in and even our body matter.
What is Carbon?
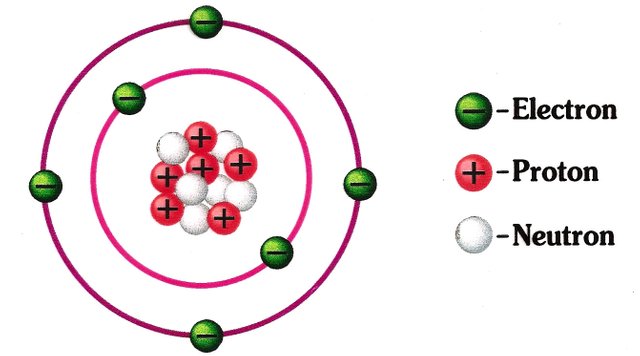
Carbon Atoms have eight electrons which participate in chemical reactions.
Carbon is a nonmetallic element classified under the group 14 and period 2 of the periodic table. It exist naturally in form in diamonds, graphite granite etc and in combined state such as carbon IV oxide, carbonic acids, methyl etc.
Carbon is the 15th most abundant element of the earth crust of which oxygen, silicon and aluminium are the highest. It is the sixth most abundant element in the universe and also the second most abundant element in the human body.
Carbon has the ability to form long resilient chains of atoms, and as well bind with up to four other atoms; This is what enables it to take part in really complex reactions and also form extensive numbers of substances; nearly 10 million compounds of carbon have been discovered. Carbon is found both in it pure form and combined as part of our physical environment as well as the biological environment. It is the building block of the CO, Hormones, Proteins, Lipids, Hemoglobin, Furniture, Cotton, etc
Physical and Chemical Properties of Carbon
| Chemical Formula | C |
|---|---|
| Melting Point | 3, 652 oc |
| Boiling Point | 4, 827 oc |
| Isotope | 3 |
| Oxidation States | +4, +3, +2, +1, 0, -1, -2, -3, -4 |
Isotopes of Carbon
Isotopy is the existence of an element with different proton number but constant neutron number hence, these elements have the same atomic number but different mass number. Isotopes are as a result of radioactive decay of the element. Isotopy however do not have effects on chemical reactions of the isotopes; they still have the same chemical characteristics.
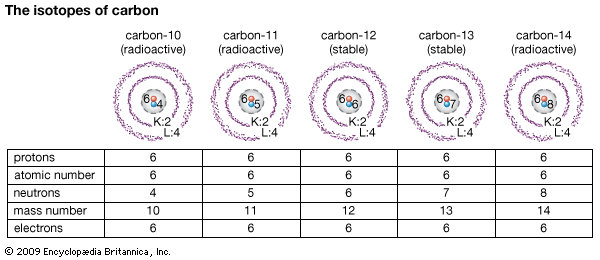
Analysys of different isotopes of carbon, C-10, C-11, C-12, C-13, C-14
There are fifteen known isotopes of carbon of which 12 C and 13C are stable. Isotopy in carbon are essential in radiometric dating . This is a method in which age of fossils are been estimated. Carbon-14 is the carbon studied for radiometric dating has a half life of 5, 740 years; It can be used to date materials as old as 50, 000 years.
Allotropes of Carbon
The arrangement of Carbon atoms is what differs in the allotropes of carbon making them have various physical appearances and other properties
Graphite is the is softest natural material known while the hardest natural material is diamond. Both are pure compounds of Carbon and they contain no other element apart from carbon. The difference in there physical characteristics is due to the arrangement of their crystal structures: This phenomenon is referred to as Allotropy.
Allotropy is the existence of an element in different structural morphology (forms), possibly different physical form and can as well attain different chemical nature. This is because the atoms of the substance are bonded in different fashion. The three major naturally occurring allotropes of carbon are:
- Diamond (They are treasured gem stone and are of more value compared to gold. It has a tetrahedral bonding of carbon atoms. This is responsible for the strong feature of the allotrope)
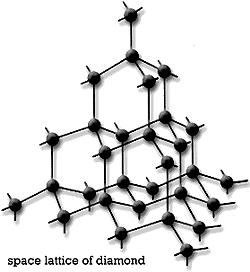
The tetrahederal arrangement of carbon in a diamond

Raw diamond before processing
- Graphite (They exist in planar layer structure. The carbon atom are bonded covalently and bonded in an honeycomb lattice in each layer. Each layers however is connected to the next by weak van-der-waals bond. This causes it to separate easily. They are found in batteries and pencils)
The layers of covalently bonded carbon atoms are attached to each other by a weak van-der-waal bond
Pure Graphite, black in apearance and breaks easily
Comparism of the arrangements of Carbon in Diamond and Graphite
- Armophous Carbon (This does not exist in crystalline form, it exist as powders and a main constituent of charcoal, activated carbon, etc)

Amorphous Carbon has bits of Diamond and Graphite in them
Changes from one form or allotrope of carbon to another can occur in the presence of light, temperature and pressure. In the industry, diamonds are produced by applying high temperature and pressure.
Carbon and Life
Growing up as a child, I used to wonder about many components of the nature such as how the sun rises, why the star are visible some night and absent in other, how it rains as well as what makes the differences between myself and the non-living things. I used to think angels poured water in a big sieve in the sky to cause rainfall, but my times of ignorance were coming to an end: I grew up and joined the science class.
We were taught about energy in physics, atoms in chemistry and cells in biology. In science class, I discovered that the same components that makes up the furniture also makes my body. Both living creatures and non-living creatures are made up of energy and atoms. Then my question on what makes me different from the furniture and the stones in my compound.
Human as well as other non-living things are made up of molecules and atoms, We often even share certain compounds such as glucose, alcohol, hydrochloric acid, iron etc.
The answer to the difference between the two creatures is metabolism.
Metabolism is a set of reactions that occurs within the cells to keep it alive. As far as metabolism is taking place, a cell, or even the entire body is said to be alive.
The three major essence of metabolism is:
- To convert food to run cellular activities
- To convert food to building blocks of substances needed for life
- To eliminate wastes, especially nitrogenous waste.
Metabolism involves breaking down and building of substances in the body, it is a continuous set of reactions that continue through out life. Hence we can say our body is a mobile laboratory. We living things basically take up (eat, consume) non-living things and living things to keep us alive as well as use their atoms and molecules to replace the atoms and molecules that make us up). Like they say, we are actually what we eat.
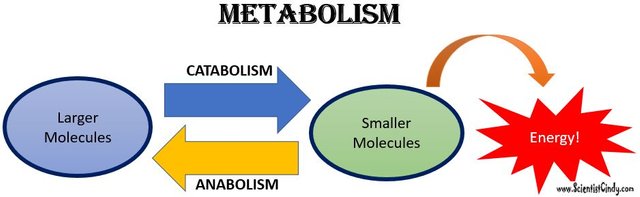
Catabolism is the breaking down of substances while anabolism is the building up. These processes either produce energy or use it up
When talking about life, we can't escape mentioning the functional unit of life; cell. It is made up of lipids, carbohydrates, proteins and water. These substance are in constant chemical reactions to keep it alive. Substance that can prevent the ongoing metabolic process are term toxic and eliminated from the body as soon as possible. This keeping the body in check is known as homeostasis.
Majority of these reactions of metabolism are as a result of the atom carbon that has the ability to exercise in deep and complex reactions as well as producing variety of substance. Catenation, the ability of carbon to form long chains give it this edge, and it has the ability to store high amount of energy. Compounds of carbon found in life are called organic-compounds. It is so amazing how carbon can be toxic or helpful depending on the elements it is combining with and the arrangement of the structure. Acids
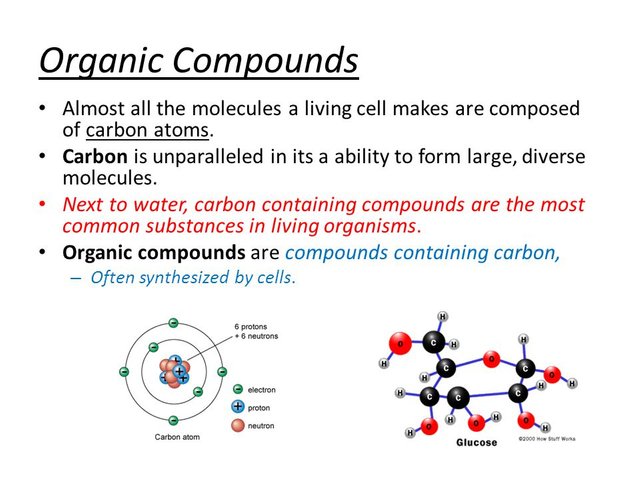
Carbon Atoms Make Up Organic Compounds of Living Organisms
It is believed that if the existence of life outside earth is true, then their body will also be composed of carbon.
What other elements are involved in life
Carbon is not the only element involved in life. Carbon react with oxygen, hydrogen, nitrogen and other substances to produce organic compounds for life. These compounds come in variety of structures and differ in functions. Examples of substances are ethanol, glucose, amino-acids, lipids, carbohydrates, hormones etc.
Further Reading:
- Live Science: Carbon
- Wikipedia: Carbon
- Wikipedia: Allotropes of Carbon
- Wikipedia: Carbon based life
- Wikipedia: Allotropy
- Wikipedia: Isotopes of Carbon
- Wikipedia: Metabolism

Thanks for taking your time to read this post on Carbon and the Mechanism behind life, I'm sure you learnt a lot from this article.
Do leave a comment on what you think about creating life from scratch using nature's resources.