Carbon Monoxide Poisoning | The Silent Killer
My today's post was inspired on my way back home from an impromptu vacation in the early hours of today. I was in a public transport when an old car speed past us with fumes of dark and smelly smokes. It clouded the atmosphere so much so our driver had to park the vehicle for lack of vision.
My post will be on a well gas known gas called Carbon Monoxide, also known as the "silent killer".
What is Carbon Monoxide?

CO is an odorless and dangerous gas, not detectable by smell
Carbon Monoxide is a colorless, odorless, tasteless gas. It is produced when substances are burned in limited availability of oxygen. Carbon monoxide has two atoms; Carbon and Oxygen with a triple covalent bond binding them. It has the chemical formula CO. Other names of the gas include carbonyl, Flue gas and Carbon II Oxide.
Production of Carbon Monoxide
Carbon Monoxide is produced from partial combustion of carbon containing substances. In the presence of sufficing oxygen, carbon containing substances such as coal, wood and ethanol burn to produce Carbon IV Oxide (CO2) and Water (H2O), this is not so in deficiency of Oxygen, Instead of producing CO2 only, CO is also produced.
Equation of A Complete Cumbustion of A carbon containing compound (Propane) in presence of Oxygen
Propane + Oxygen --> Carbon IV Oxide + Water
Equation of an Incomplete Combustion of A Carbon containing compound (Propane) in absence of enough Oxygen.
Propane + Oxygen --> Carbon + Carbon Monoxide + Carbon IV Oxide + Water
Sources of Carbon Monoxide
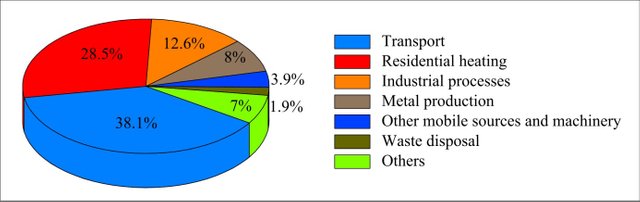
Most of the CO generation is from our transport system
If you are on a journey within Nigeria, from Lagos to Osun; you definitely will perceive the difference between the constituent of the atmosphere in Lagos, Ibadan and Osun States. This is because Lagos being a commercial center has a lot of vehicles producing this dangerous gas, Carbon Monoxide. Even almost every night, 80% of the household put on standby generator(Of which majority is a breed of light generator known as "I pass my neighbor"). This is why most of the cases of carbon monoxide poisoning in Nigeria is from Lagos.
Common causes of Carbon Monoxide include sources of combustion such as:
- Stoves
- Ovens
- Fire Places
- Water Heaters
- Space Heaters
- Portable Generators
- Charcoal grills
- Clothes dryers
- Locomotive Trains
- Aircraft
- Vehicles such as Cars, Lorries, Buses, Motorcycles, Tricycles, Scooters etc.
In presence of sufficient oxygen to aid complete combustion and proper functioning, some the appliances above produce Carbon Monoxide in minute quantities not taggable as dangerous.
Carbon Monoxide, Hemoglobin and Myoglobin
Oxygen is transported to the cells of the body by the hemoglobin of the red blood cell, this is essential so that aerobic cellular respiration can take place. In absence of oxygen in the cells and tissue, there is production of excess lactic acid causing acidosis, less energy production in the body, unconsciousness, fainting and possibly death. Hypoxemia, low oxygen level occurs when there is adequate atmospheric air or ventilation, and in some cases, the inability of oxygen to bind with hemoglobin.
Carbon Monoxide has higher affinity for hemoglobin than oxygen. Carbon Monoxide act with hemoglobin to form Carboxyhemoglobin. The affinity of Carbon Monoxide for hemoglobin is about 200 times more than that of oxygen. This causes the hemoglobin to transport carbon monoxide instead of the intended oxygen gas.
Reaction equation showing normal reaction in absence of CO
Hemoglobin + Oxygen --> Oxyhemoglobin (Bright Red Color)
Reaction equation in presence of CO
Hemoglobin + Carbon Monoxide --> Carboxyhemoglobin (Cherry-Red Color)
This reaction is similar when myoglobin is exposed to carbon monoxide. Myglobin is a similar iron-oxygen binding pigment like hemoglobin. It is found in muscle tissue and responsible for their red coloration. It is responsible for taking up oxygen from hemoglobin and releasing to muscle cells. Carbon monoxide is intentionally added to meat to give it a red coloration(More details in function of carbon monoxide).
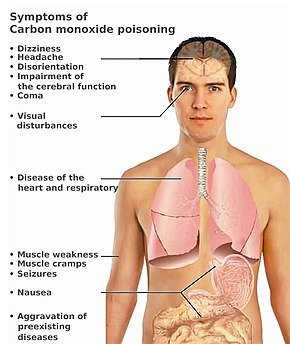
Symptoms of Carbon Monoxide Poisoning
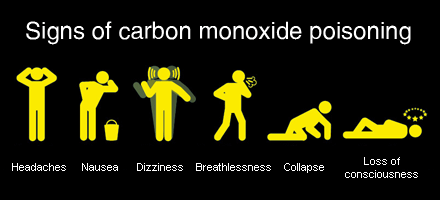
Gradual Symptoms of Poisoning
Carbon Monoxide no taste, smell or color hence, people cant detect when they are exposed to the gas. The gas has the ability to make one unconscious within 2 minutes and lead to death in 3 minutes of exposure. The following symptoms are however experienced in different quantities one is exposed to. One is exposed to gas wont testify to any of this symptoms till the person dies from its poisoning. Below are the symptoms:
- Headache
- Dizziness
- Fatigue
- Nausea
- Increased heart rate
- Seizures
- Loss of consciousness
Carbon Monoxide tolerance

Babies are less tolerable to the Gas. Men are more tolerable but there is more record of men dying to the poising than women
There is variation of tolerance among people of different age, age, sex, genes etc. Babies are more likely to die from the same quantity of Carbon Monoxide needed to make an adult vomit and nauseating, this is because they not only have less red blood cells in their body but a smaller body ratio. Tolerance also depends on rate of ventilation, activity level, underlying blood disorder such as sickle cell anemia, barometer pressure and metabolic rate.
Effect of Carbon Monoxide Poisoning
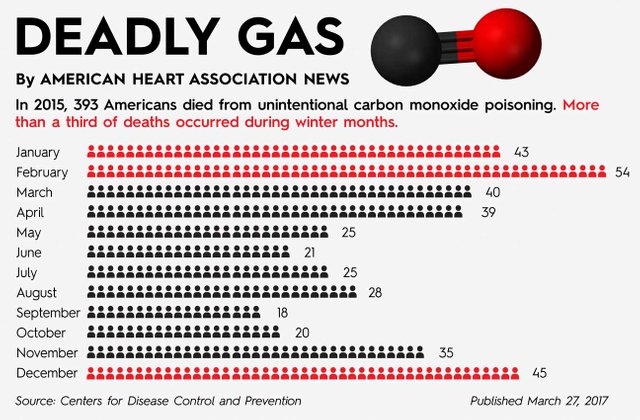
Almost every day of the year, people die of carbon monoxide poisoning
Exposure to smaller concentration of carbon monoxide over a period of time can deteriorate health of an individual. The systems of the body most affected by this poisoning are the organs that require oxygen the most: the brain and the heart. The brain sense reduction in oxygen level of the blood (hypoxemia) and signals the heart begins to pump blood faster in hope to get oxygen; this indirectly accelerates more intake of carbon monoxide leading to death.
The brain in absence of oxygen might do into hallucination stage or just go unconscious. Other organs and systems of the body affected by this poisoning include:
- Respiratory System
- Renal System
- Gastrointestinal System
- Muscular System
Diagnosis of Carbon Monoxide Poisoning
The symptoms of carbon monoxide is similar to that of infections such as flu, as ectopic pregnancy and appendicitis share the similar symptoms. However, knowledge about one's exposure to fire source can guide the diagnosis of carbon monoxide poising, poisoning is confirmed by measuring amount of carboxyhemoglobin in the blood.
Ratio of carboxyhemoglobin in a normal person is about 5%; Carboxyhemoglobin is present due to release of Carbon Monoxide during catabolism of heme from hemoglobin by Heme Oxygenase and not as a result of carbon monoxide exposure. In smokers, this ratio can go as high as 9%. Most people who die of carbon monoxide poising had ratio of 30 - 90%
Treatment of Carbon Monoxide
The first aid given to suspected victims is to evacuate them out of source of the deadly gas. You are expected to take heed not to get poisoned yourself.
In the presence of emergency team, unconscious victims could be given a cardiopulmonary resuscitation at the site. Non-rebreather mask could also be used to administer oxygen to the patient.
Does Carbon Monoxide Exist Naturally?
Biologically and in our Natural physical environment, Carbon Monoxide is produced. Common source of this gas are:
- Volcanoes
- Natural Forest Fires
- Mines and Caves (Miners refer to it as white damp
It is produced in the body of human and animal during break down of heme from hemoglobin. This is a normal physiological process.
Home detection of Carbon Monoxide
This is one of the preventive methods of carbon monoxide poisoning. Carbon Monoxides detectors are used in detection of indoor carbon monoxide. It works like a smoke alarm and should be placed in every room including the toilets and kitchens.
But Carbon Monoxide is not that bad, it has it's own functions:
You might be shocked to know that this gas that has claimed a lot lives also has its own benefits. In industries, it is used as a precursor of many products such as aldehydes(these include many products including perfumes), phosgene (used in synthesis of pharmaceuticals and organic compounds), methanol (fuels for vehicles) etc.
It is also used in the food industry for meat coloration. The mechanism involved in this process is simple, the carbon monoxide combines with the meat myoglobin to form a bright-cherry-red pigment, carboxymyoglobin. This make the meat, fish or pork look more fresh.
Carbon Monoxide is used in medicine as vasodilaors, in metal industry to purify metals from their ores as well as in production of infrared lasers.
Further Reading:
- Mayo Clinic
- Wikipedia: Carbon Monoxide
- Wikipedia: Carbon Monoxide Poisoning
- Carbon Monoxide Chemistry
- Wikihow: Detection of Carbon Monoxide
- Everyday Health: Detection of Carbon Monoxide
- Wikipedia: Carbon Monoxide Detection
- Vanguard: Death caused by Carbon Monoxide
- Heme Oxyganase: Catalyst for hemoglobin breakdown
- Wikipedia: Carboxyhemoglibin
- Hypoxemia: Low blood oxygen level
- Carbon Monoxide, Myoglobin and Hemoglobin

Thanks for taking your time to read this post on Carbon Monoxide, I'm sure you learnt a lot from this article.
Do leave a comment on what you think about eradicating combustion vehicles and replacing them with more bio-friendly electric ones



Being A SteemStem Member