Observing phenomena affecting solubility: Common Ion

The chemistry contemplates very interesting topics, daily you can observe the solubility of different compounds, such as common salt (sodium chloride), sugar (sucrose) or any other similar compound. However, not all compounds have high solubility, so some are called insoluble compounds. Solubility is a very relevant concept for chemistry, with very diverse applications; from the use in the industry of many factories, to marketing campaigns for the advertising of a new product, for example, a detergent. In this post, the influence of a common ion in solution, calcium hydroxide (Ca(OH)2) and calcium chloride (CaCl2), will be presented in two experiments; they are compounds containing calcium (Ca), therefore, both being in one solution, they change the solubility balance of the little soluble compound, in this case calcium hydroxide. This phenomenon not only occurs with these compounds, but also in nature quite frequently.
Theoretical Basis of the Common Ion Effect
Some compounds such as NaCl have a relatively high solubility, other compounds have a low solubility, the part that dissociates in water may even be negligible, so these compounds are referred to as insoluble or slightly soluble.
These compounds have an associated constant of ionic products, or "Ksp", which is defined as the product of the molar concentrations (in equilibrium) of the constituent ions, each of which is elevated to the power of the stoichiometric coefficient in the balanced equilibrium equation. Given a general dissolution, it follows that the Ksp will be:
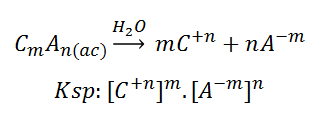
In this way, the solubilized part of a slightly soluble compound is calculated, taking into account its constant Ksp (experimentally determined for a given solvent, temperature and pressure). An example might be calcium hydroxide:
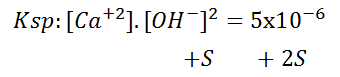
Where the solubilised part is represented by the letter 'S' together with the corresponding stoichiometric number, its Ksp:
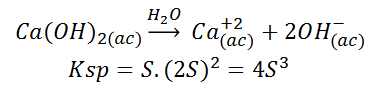
One effect that causes a decrease in the solubility of a compound is the common ion. When a compound is added to provide the solution with an ion in common with another compound that is not very soluble, the equilibrium is shifted to the left by the principle of Le Châtelier, increasing the part of the compound that remains undissociated and thus the solubility decreases. The Ksp value of the species remains unchanged.
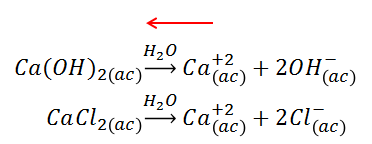
In this case the common ion is Ca+2, which decreases the solubility of the insoluble compound.

In order to verify the above, an experimental procedure will be presented below, which will compare qualitatively the presence and absence of a common ion and its effect on the solubility of a slightly soluble compound:
Solubility of calcium hydroxide in water.
In a 250ml beaker a saturated solution of Ca(OH)2 was prepared with approximately one gram of Ca(OH)2 and 150ml of distilled water. It was gently mixed to allow the maximum possible amount of solute to dissolve, and left to settle so that the non-differentiated solid could settle.
The temperature was taken and the solution was filtered by the simple bending method, always covering the solution to avoid possible carbonation.
Then with a standardized solution of HCl at 0.0488 M the system was prepared to titrate the previously filtered solution, using bromocresol green as an indicator (which has a yellow to blue shift in the range 4.04-5.6 on the pH scale). A total of 4 times 25ml aliquots of the filtered solution were titrated.
.
Solubility of calcium hydroxide in water in the presence of CaCl2 (common ion)
In a 250ml beaker a solution containing approximately 1.5 grams of calcium chloride (CaCl2) and one gram of calcium hydroxide (Ca(OH)2) was prepared, along with 150 ml of distilled water. It was gently mixed while each compound was added slowly and in the order indicated above to allow the maximum amount of solute to dissolve, and allowed to stand so that the non-differentiated solid could settle.
The temperature was taken and the solution was filtered by the simple bending method, always covering the solution to avoid possible carbonation.
Then with a standardized solution of HCl at 0.0488 M the system was prepared to titrate the previously filtered solution, using bromocresol green as an indicator (which has a yellow to blue shift in the range 4.04-5.6 on the pH scale).
Knowing that the solubility of calcium hydroxide at 25° C is 0.167 g in 100 ml of water, as the aliquots are 25ml, then the solubilized part corresponds to 0.0418 g. (value taken from CRC HandBook of Chemistry and Physics, 86th Edition, CRC Press 2005)
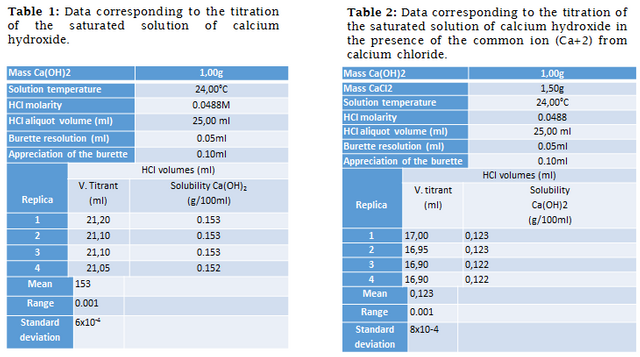
According to the titration, the titrated moles of oxydryl ions correspond to 1,030(6)x10^(-3) mole on average.
According to the dissociation reaction of the compound, the solubilized part of the oxydrile ion is double stoichiometrically to calcium hydroxide, so the moles corresponding to calcium hydroxide are:
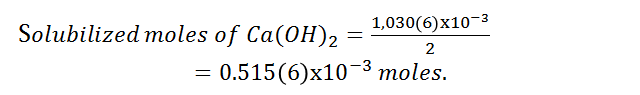
Being the grams of calcium hydroxide solubilized:
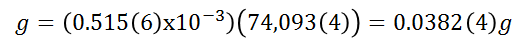
This quantity corresponds to the solubility in 25ml of water, similarly for 100ml the solubility will be: 0.153(4) g/100ml. This quantity represents 91.39% of the theoretical quantity for 25°C. It is important to note that the temperature at the time of the practice was approximately 24°C, therefore, these temperature variations (since it could later increase) could systematically affect the measurements performed.
Analogous to the above, it can be calculated, according to the data of table 2, that the solubility value in this experience is 0.122g/100ml.
This quantity represents 73.21% of the theoretical solubility in 100ml of water, at 25°C and with the presence of a common ion. The decrease in the solubility of the compound after the addition of Ca+2 as a common ion is evident.
Conclusion
Based on the results obtained, it was possible to quantitatively calculate the effect of the common ion on the solubility of Ca(OH)2 which decreased by approximately 20%.
As the solubility is relative to the temperature, this could be a source of systematic errors since it was not constant, and although it was not 25°C, it could vary in the course of practice, thus influencing the solubility of the compound is a question.
In synthesis, the titration technique made it possible to calculate the concentrations of the oxidryl ions, and then to calculate the solubilized grams of the compound, using stoichiometry and its molecular weight. Even with some associated systematic errors it was possible and satisfactory to observe how much a common ion affects the solubility of a compound.
I hope that this brief theoretical explanation allows us to understand that the solubility of a compound depends on many factors, including the solvent, but also on the other components found in it. This is one of my favorite effects, and I think it manifests itself many times in experimental chemistry as well as in nature. Thank you for reading.
References:
All the images of my authorship were edited and processed using the software PowerPoint 2016.
- Theodore Brown, Eugene LeMay, Bruce Bursten, Julia Burdge, (2004), Chemistry. The central science. (9th Edition). Pearson Education, S.A., Chapter 17.
Douglas Skoog, Donald West and James Holler (2006) Fundamentals of Chemistry Analytical (4th Edition) Editorial Reverté.
CRC HandBook of Chemistry and Physics, 86th Edition, CRC Press 2005

Posted from my blog with SteemPress : http://aleestra.vornix.blog/observing-phenomena-affecting-solubility-common-ion/
This post has been voted on by the steemstem curation team and voting trail.
There is more to SteemSTEM than just writing posts, check here for some more tips on being a community member. You can also join our discord here to get to know the rest of the community!
Hi @aleestra!
Your post was upvoted by utopian.io in cooperation with steemstem - supporting knowledge, innovation and technological advancement on the Steem Blockchain.
Contribute to Open Source with utopian.io
Learn how to contribute on our website and join the new open source economy.
Want to chat? Join the Utopian Community on Discord https://discord.gg/h52nFrV
Congratulations @aleestra! You have completed the following achievement on Steemit and have been rewarded with new badge(s) :
Click on the badge to view your Board of Honor.
If you no longer want to receive notifications, reply to this comment with the word
STOPDo not miss the last post from @steemitboard:
SteemitBoard and the Veterans on Steemit - The First Community Badge.