The Wave Nature Of Light
Light

Wave Nature Of Light
When a light consisting of a certain element is switched on, the electrons of that elemental atom are excited to a higher energy level, and this process is observed due to the addition of electricity. This excited state however, is undesirable for the atom and thus, the atom attempts to return to its lower energy level, such as the one before excitement occurs. In the process of returning to its an excited state, energy is released by the atom. This energy is what we refer to as light. This result is known as the most important discovery in the 20th century and is referred to as the quantum theory. But for this post, I will not be explaining this theory, but will instead explain the fundamental aspect of this theory, which is light. Light is a discovery that we take for granted every day in our lives. We rely upon this for many applications as well as to overcome challenges that we faced in the past, therefore, in this post I will be covering the wave nature of light.
To understand light, scientists had to analyse the electronic structures of atoms as these components of an atom would result in either the omission or absorption of light by these substances. The light that we view through our eyes is what we refer to as visible light, and this form of light is an example of electromagnetic radiation, which is the oscillation of electric magnetic fields. An important point to mention, is that visible light is not the only form of electromagnetic radiation there is, as there are many different forms of light, such as:
- Radio waves
- Infrared radiation
- X-rays
- Microwaves
- Gamma rays
- And so on,
All electromagnetic radiation share similar fundamental characteristics. The most important features that is common to all is the speed at which the radiation moves through a vacuum. This speed is equivalent to the speed of light, which is 3 x 10^8 m/s. This is an important discovery, as it indicates that a wave is one of the fastest known entities in the cosmos, as space is a vacuum. A secondary feature that is persistent in all forms of electromagnetic radiation is the occurrence of extremely similar wave-like properties to that of a wave passed through a body of water. Since the energy of waves through water express an up and down movement of water, it indicates to us that these waves oscillate energy through motion.
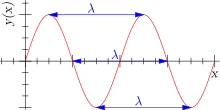
It can also be noted that it indicates a periodic pattern of peaks and troughs in repeated intervals. In a waveform, the distance between two adjacent peaks or two adjacent troughs, is referred to as the wavelength. The wavelength is used to calculate the number of cycles of a wave, where a cycle is the summation of all wavelengths present. The idea of a cycle can then be related to a term known as frequency, which is the number of cycles that pass through a given point per second.
Since water waves are similar to electromagnetic waves, the preceding terms can be calculated for electromagnetic waves also, but, there is a difference however. We must recall that electromagnetic radiation moves at the same speed throughout it’s duration. Due to this reason, wavelength and frequency of electromagnetic radiation is always related in a straight forward motion as the speed of light is in the same direction.

Water Waves: Macroscopic Analysis
When scientists investigated the analysis of waves, a few trends and observations were noted. The most important of these trends were those that related the wavelength to that of the frequency, as it is these properties that affect the electromagnetic spectrum. It was noted that if the wavelength of a specific wave is long, it would subsequently results in fewer cycles of a wave passing through a single point in a single second, thus indicating that the frequency of that wave is low. Alternatively, the wavelength of that identical wave is short, the frequency will subsequently be larger. The above relationship is what scientist refer to as and inverse relationship, which can be expressed as:
v=c/λ where,
v = frequency
c = the speed of light, a constant (3 x 10^8 m/s)
λ = wavelenght
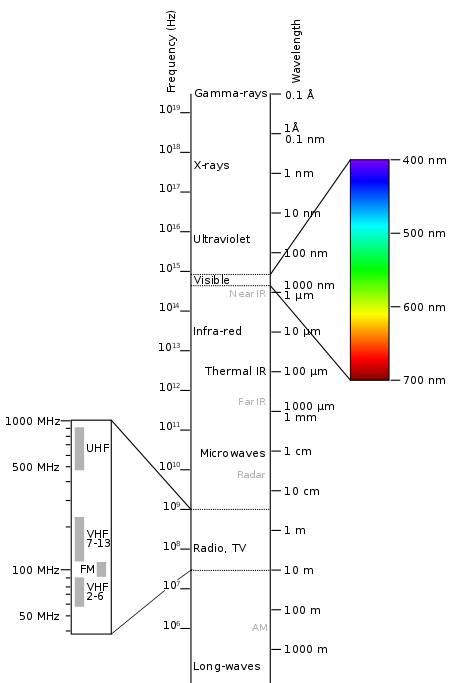
As aforementioned, the difference in wavelength of certain waves will produce different properties in those respective waves. These differences is what we refer to as the electromagnetic spectrum, where a range of wavelengths are categorised in order of wavelengths, for us to identify that of visible light and those of other branches of light. It can be noticed that the range of wavelengths extend through a large variety. The wavelength of gamma rays can be noted to be similar to the diameters of their atomic nuclei, whereas, the wavelengths observed by radio waves and light of longer wavelenghts can often be longer than that of a sports field.
The discovery and development of the electromagnetic spectrum is of great importance to us as this information helps us observe as well as analyse the composition, as well as the temperature or even the velocity of a body that emits or absorbs light. Although we may now realise of its importance, we should give thanks to the scientist that spent decades trying to develop the spectrum. Each portion of the spectrum consists of a different form of wave, which were subsequently found by different scientists in different years.
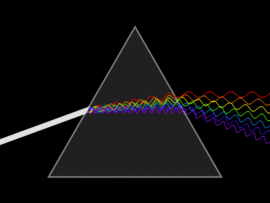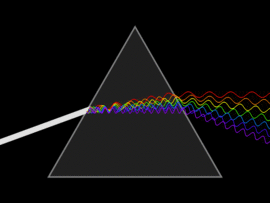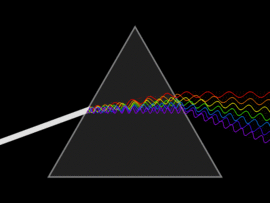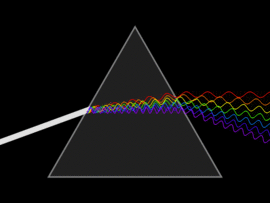
The discovery of infrared light took place in 1800, by a scientist by the name of Sir William Herschel. In his attempt to analyse the amount of heat contained in different colours (in the range of visible light), he unknowingly discovered infrared light. By passing sunlight through a glass prism, he successfully separated the sunlight into the seven colours of the rainbow. He then placed a thermometer within each colour range and also placed another that was out of the spectrum (more accurately, past the red light). In doing so, he observed that the thermometer that was placed outside of the visible light spectrum had a higher temperature than compared to the thermometer that was placed within the specific ranges. This experiment gave rise to infrared light.
In 1801, a fellow scientist by the name of Johann Wilhelm Ritter was inspired by the aforementioned scientist, and conducted an experiment to investigate whether light was present beyond the purple terminal of the spectrum. When the experiment proved successful, ultraviolet light was discovered. Heinrich Hertz demonstrated that waves with a larger wavelength than that of infrared light existed, as in 1887, he developed radio waves in his laboratory.
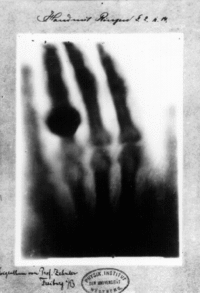
The discovery of waves with a shorter wavelength than that of infrared light happened in 1895 by a German scientist known as Wilhelm Conrad Röntgen. The discovery of x-rays was by accident, as Röntgen was experimenting with vacuum tubes and uncovered an unknown form of light. In the proceeding weeks of discovery, Röntgen took a picture of his wife’s hand using this new form of light. The result of this picture was quite astonishing as it not only revealed the bones in his wife’s hand but also an indication of the jewellery (a ring) that she was wearing. The initial name to this form of radiation was called “X”, to which it was later referred to as X-rays.
Paul Villard was the scientist responsible for the discovery of gamma rays, while investigating radiation from an element known as radium, in 1900. This form of light was known to reflect from layers of crystal and thus, were noted to have comparable similarities to that of x-rays. An added property of gamma rays is that this form of light has a much shorter wavelength than that of x-rays.
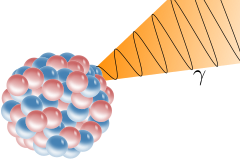
Gamma Radiation from the nucleus of an atom
This brings us to the end of my post on the wave nature of light as well as an introduction to the electromagnetic spectrum and the timeline of discovery. The electromagnetic spectrum, till the present day, is used in many industries and is even noted to be used in analysis of the vacuum of space. We should be highly thankful to the years of research undertaken by scientists of the past to allow us to live our lifes in the way we do today.
Images are linked to their sources in their description
The End
References:
[1]https://en.wikipedia.org/wiki/Wave
[2]https://en.wikipedia.org/wiki/Waveform
[3]https://physics.ucsd.edu/students/courses/summer2011/session1/physics2c/Waves.pdf
[4]https://en.wikipedia.org/wiki/Visible_spectrum
[5]Chemistry,The central science: A broad perspective
[6]https://imagine.gsfc.nasa.gov/science/toolbox/history_multiwavelength1.html
[7]https://www.independent.co.uk › News › Science

Being A SteemStem Member
Nice post. I just have some issue with the first sentences...
If I may rephrase it, light carries energy. If one bombards some atoms of a given species with light of a very specific energy, those atoms will get excited. It is also more correct to speak about electromagnetism than electricity.
All along the post, what you call electric is in fact electromagnetic.
Thank for the correction. I see that that reconstruction makes it easier to understand and more scientifically correct. Duly noted with respect to the misuse of electric, I will make sure it does not resurface in my posts related to that of light. Thank you for bringing this to my attention :)
The pleasure was for me :)
valuable post on electromagnetic technology..
thanks for sharing..
Thank you for taking the time out to read my post :) i really appreciate it :D