Acids and Bases
Acids and bases
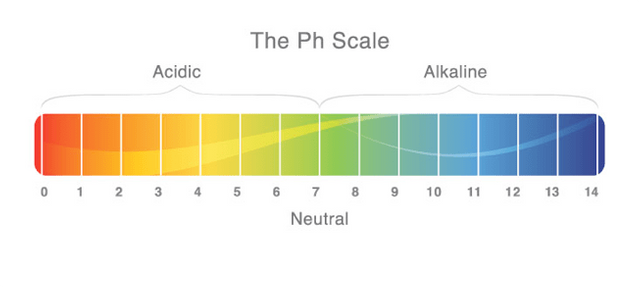
In chemistry, it is often said that acids are the chemical opposite of a bases and vice versa. We tend to define acids as compounds of matter that tend to “donate” a hydrogen ion (H+) to another compound. The compound that receives the ion is what we refer to as a base. The word acid originates from a Latin descent of the word "acidus" or "acere" which translates to sour. Scientifically speaking, an acid is any chemically compound that produces a solution with an increased hydrogen ion concentration that that of pure water. Since water has a 7.0 rating upon the pH scale (percentage hydrogen), and due to an acid adding ions in solution, the pH of an acid is between 0.1-6.9 where a strong acid is closer to 0 while a weaker acid is closer to 7.0. Since we understand that a base is the chemical counter of an acid as previously mentioned, a base is a chemical compound that produces a solution with a decreased hydrogen ion concentration and with a pH between 7.1-13.9 where a strong base is closer to 14 and a weak base is closer to 7. It must be noted that although a pH scale runs from 0-14, we only give values to everyday acids and bases between 0.1-13.9. the reason for this is because the values 0 and 14 represent ideal acids and bases which can only occur under ideal conditions (25° C, 1 atmosphere of pressure).
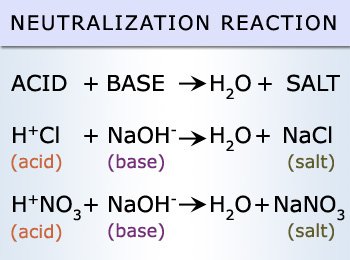
These values are unlikely to occur naturally unless in a closed environment, therefore we may never commercially come across and acid and a base of values 0 and 14 respectively. A reaction between an acid and a base is referred to as a neutralization reaction. This reaction will result in the formation of 2 products, water and a salt.
Strong acids are pronounced from having a high corrosive effect on metals. This reaction can take place with most metals and a by-product of this reaction is Hydrogen gas. The gas can be either disposed of safely or can also be sold commercially to or by licensed gas retailers. Bases on the other hand, have a strong effect on organic matter. Both, acid and bases, are extremely versatile in everyday life and have a variety of uses. Acids are used for the removal of rust from metals, produces gasoline and other gases (as mentioned), used in the processing of many minerals and even used as an additive in our eating and drinking products. Bases have many domestic uses as they are used in many home cleaning products such as detergents for dishwashing and laundry as well as over cleaners and stain removal items.
Many form of acids exist, such as sulfonic acid (contains Sulphur as the main constitution) as well as carboxylic acids (contains carbon, oxygen, hydrogen and forms the formulae -COOH), nucleic acids (found in DNA) and so on. H¬¬2SO4 (sulfuric acid) and H−CH2COOH (acetic acid) are examples of the prementioned groups respectively.
Acids and bases can also be split into 2 groups, organic or inorganic:
An inorganic acid is an acid that has a compound of Hydrogen and one or many elements (except for carbon) that, when dissolved in water, breaks down the compound to produce hydrogen ions. This resulting solution has the ability to neutralize a base, spark a change in the colour of litmus paper as well as production of certain colour changes with the use of indicators. Inorganic acids are often referred to as mineral acids. Inorganic acids are used as chemical catalysts during reactions and can be found in many industrial factories, such as metal making, petroleum, textiles and even photography. A few examples of inorganic acids are hydrochloric acid, sulphuric acid and even nitric acid.
Hydrochloric acid (HCl) is used for acidizing in industry, the refining of mined tin and tantalum for ore, conversion of corn-starch to syrup and even used under intense heat situations in boilers and heat exchangers.
Sulphuric acid (H2SO4) is used in the parchment of paper as well as the purification of petroleum products, extracting uranium from pitchblende, pickling of iron and steel, and even in the processes involving the refining of vegetable oils. Another acid that belongs to the sulfonic and inorganic class is Sulphamic acid (H3NSO3) is optimized in the wood and textile industry as a method of flame protection of the products. Other uses such as in the paper and pulp industry have been recorded as sulphamic acid acts as a good bleaching agent. It is also used to control and stabilize the amount of chlorine in swimming pools.
Nitric acid (HNO3) is mainly used in the production of fertilizers and explosives as they both contain ammonium nitrate which is manufactured from nitric acid. It is also used in metallurgy, ore flotation upon mining environments and in nuclear fuel.
The following list gives us a larger range of products of organic and inorganic acids and bases.
Inorganic Acids:
Hydrogen Sulfide (H2S) - found in decaying animals, volcanic ashes, some mineral water, unrefined fuels, such as crude oil, coal, natural gases.
Phosphoric Acid - H3PO4
Sulfuric Acid - H2SO4
Organic Acids:
Citric Acid - found in lemons, times, oranges, pineapples
Carbonic Acid (H2CO3) - can be found in carbonated drinks
Hydrogen Cyanide - HCN
Lactic Acid - found in sour milk, yogurt, cottage cheese
Tartaric Acid - found in grapes
Inorganic Bases :
Calcium Carbonate (CaCO3) - found in calcite, aragonite
Calcium Hydroxide - Ca(OH)2
Sodium Bicarbonate (NaHCO3) - found in baking soda
Sodium Carbonate – found in seaweed ash
Organic Bases:
Ethylamine
Before understanding the behavior of acids and bases as we do today, many scientists undertook the task of finding definitions of their behavior over time. Here a few predictions made by scientists of yesteryear on the definitions of acids and bases.
- In an aqueous solution, acids produce H+ ions
- In an aqueous solution, bases produce OH- ions
- Water is required so a restriction of only aqueous solutions was observed at the time.
- Only protic acids are allowed.
- Only hydroxide bases are allowed.
• Johannes Nicolaus Brønsted - Thomas Martin Lowry
- Only the acid are proton donors
- Only the bases are proton acceptors
- Aqueous solutions are permissible
- All bases except hydroxides are to be permissible
- Only protic acids are to be allowed
- Acids are now electron pair acceptors
- Bases are therefore electron pair donors
- It must be noted that this method is the least restrictive of the acid bases definitions as there are no restrictions for the type of acids and bases to be used or even for the type of solution present at the time of reaction.
Images are linked to their sources in their description
The End
References:
[1]https://www.educator.com/studyguide/chemistry/introduction-to-acids-and-bases/
[2]http://youbasic.weebly.com/neutralization-reactions.html
[3]https://www.thoughtco.com/definition-of-corrosion-604960
[4]https://www.nobelprize.org/nobel_prizes/chemistry/laureates/1903/arrhenius-bio.html

/GettyImages-548553969-56a134395f9b58b7d0bd00df.jpg)






Nice work, a complete and simple way to describe this kind of compounds.