The Magic Of Quantum Chemistry
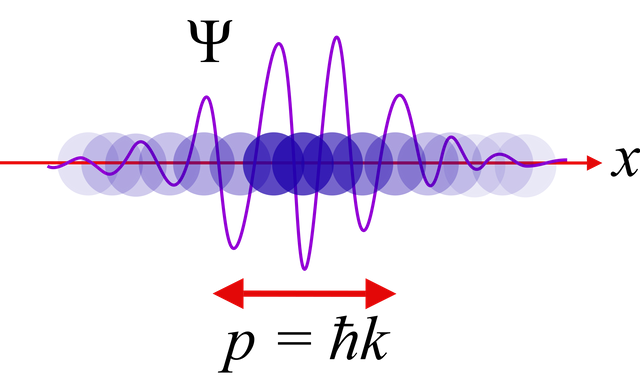
Ever since the discovery of quantum mechanics, it seems the structure of the world around us is anything but intuitive.
Let’s take a gander at our intuitive understanding of ‘solids’ for example, let’s say a rock.
A rock is probably as solid of an object as most of us can imagine.
We perceive it as a continuous structure of uninterrupted matter, extremely dense and rigid.
But is it?
Solid materials are actually constructed out of peculiar elements called atoms, and even more peculiar elements called electrons.
One famous early notion which has since been superseded, was proposed by famous scientist Niels Bohr.
The Bohr model suggested that electrons orbit around atoms, in 'ordained' motion, much like planets around a star.
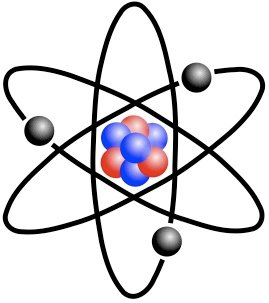
Bohr Model
Probabilities
What quantum mechanics has to teach us, is When we talk about electrons surrounding an atom, we actually talk about a ‘cloud of probabilities’.
Probabilities of what? Probabilities of where the electron is at any given moment. That’s right.
The electrons aren’t actually there, they are only probably there.
Take a look at the following diagram of 'probability densities' - the changes in color indicate how probable it would be for an electron to occupy a certain position around the atom.
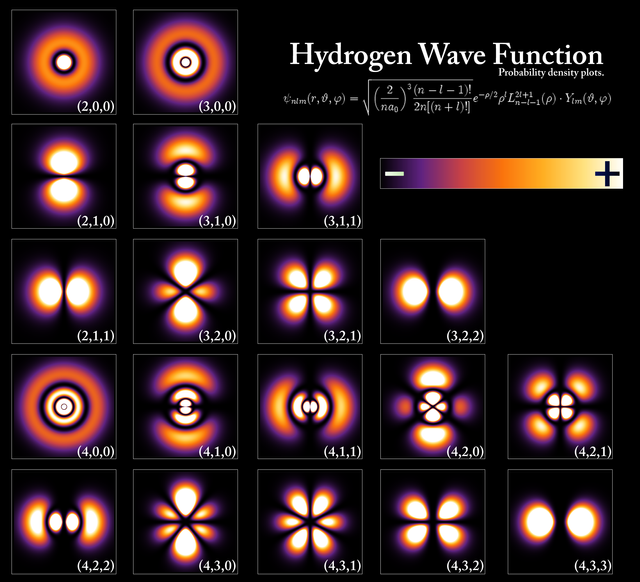
Quantum Weirdness
Not only that solid matter is not as rigid we perceive it to be, it is actually built (if you will) by clouds of probabilities surrounding minuscule entities.
These ‘clouds’ come in many crazy shapes, and are named atomic orbitals.
The geometric shapes of atomic orbitals are calculated using what is called ‘quantum numbers’ which stem from advanced mathematics, and have visual representations.
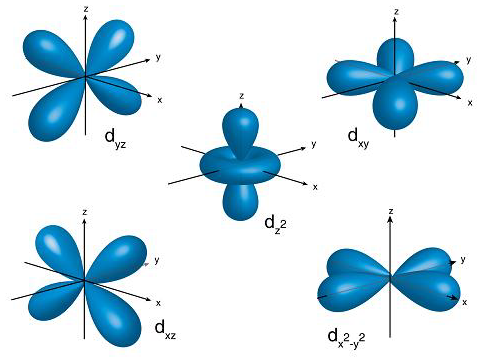
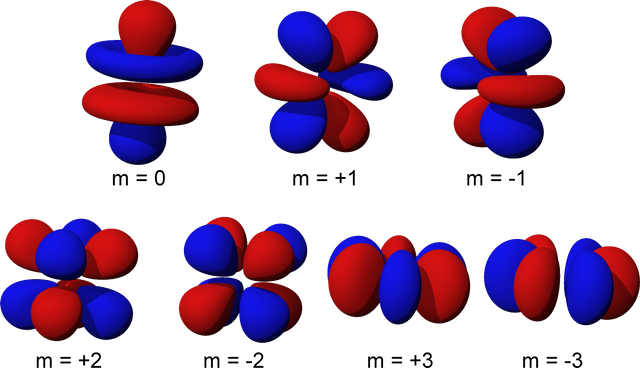
So far we have discussed a single atom with a cloud of probabilities surrounding it, but we all know that matter is constructed out of a huge amounts of these elementary particles, so what would that look like in the quantum world? Even weirder.
It seems that when atoms band together to create molecules, their ‘clouds’ combine and interact to form completely new probability fields called molecular orbitals (no surprise there).
What if we were to compare the size of an atom to the size of an electron.
Intuitively and by the classical model, the atom is bigger than the electron by orders of magnitude, but when we look at electrons as a field of probabilities, what would ‘bigger’ even mean?
Go deeper down the rabbit hole:
Quantum physics: What is really real?
Introduction to quantum mechanics
Congratulations @sifodyas! You have received a personal award!
Click on the badge to view your own Board of Honor on SteemitBoard.
For more information about this award, click here
Congratulations @sifodyas! You have received a personal award!
Click on the badge to view your Board of Honor.
Congratulations @sifodyas! You received a personal award!
You can view your badges on your Steem Board and compare to others on the Steem Ranking
Vote for @Steemitboard as a witness to get one more award and increased upvotes!