BCL-2 homology and Signal transduction in relation to mitochondrial-dependent apoptosis, and neoplasia (part 2)
2.0 BAK and BAX in relation to MOMP
2.1 BAK
BAK is located in the OMM where its C terminal α9 helix is constitutively inserted. Voltage-dependent anion channel isoform 2 (VDAC2) occupies the dimerisation pocket of BAK by interacting with its hydrophilic tail (Lazarou et al., 2010), sequestering BAK in its inactive form raising the stimulation threshold for MOMP. A study using blue native PAGE (BN-PAGE) has shown endogenous BAK existing as a 400kd complex in the OMM (Lazarou et al., 2010). This complex is shown to be VDAC2-dependent. Mutational studies involving the absence of VDAC-2 have shown that BAK exists in the OMM as an inactive monomeric form, but with a lower threshold required for activation by direct activator proteins (Kim et al., 2009: Lazarou et al., 2010). These results support that homo-oligomerisation is direct activator-dependent, and that VDAC2 essentially acts as a break regulating BAK activation.
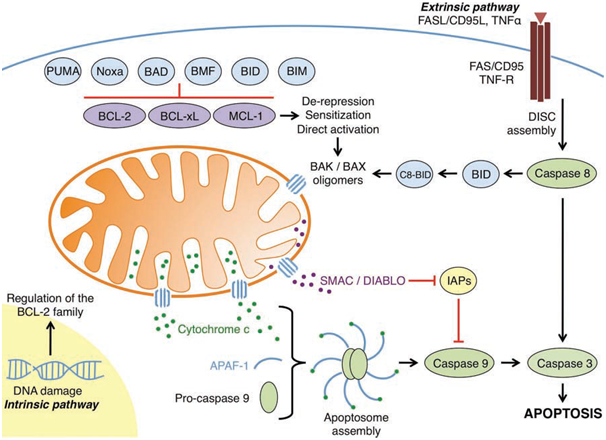
Figure 1: An overview of the intrinsic and extrinsic apoptotic pathways
(Elkholi et al., 2011).
BAK is activated by the direct activators proteins tBID, BIM, and PUMA. Noxa, another BH3-only protein has been shown to also directly activate BAK (Dai et al., 2011). Following pro-apoptotic stimulation the direct activator proteins transiently interact with BAK at its BH3 domain, causing a conformational change dissociating the VDAC2:BAK complex, and freeing BAK. Interaction between the BH3 activators and BAK is transient as the BH3 domain of BAK is required to form dimers with other BAK molecules. BAK exposes its BH3 domain and inserts it into the BCL-2 family BH3 and C-terminus-binding groove (BC groove) of another BAK monomer. Homodimerisation occurs through cysteine interactions at the BH3:BC groove (Dewson et al., 2009). These BAK dimers are then converted into large oligomers via cysteine interactions in their α6 helices, creating high order BAK oligomers made up of BAK dimers linked together by α6:α6 interfaces (Dewson et al., 2009). The α6:α6 interaction promotes MOMP but is dependent upon the BH3:BC groove interface occurring.
2.2 BAX
Unlike BAK, BAX is predominantly found in the cytosol where it exists as a monomer. BAX requires an additional activation step where upon activation it translocates to the OMM to induce MOMP. The direct activators tBID, BIM, and PUMA have been shown to directly interact with and activate BAXα (Lovell et al., 2008: Gavathiotis et al., 2008: Kim et al., 2009). The C terminal α9 helix of BAXα is kept engaged by the α1 helix in the dimerisation pocket. The amphiphatic nature of the α9 helix enables this interaction and the α1 helix stabilises it, rendering BAXα as a monomer in the cytosol. The α9 helix is required for OMM insertion. For dimerisation and translocation to the OMM to occur the α1 helix must be exposed, resulting in secondary disengagement of the α9 helix. Disengagement of the α9 helix leads to OMM targeting. The direct activators tBID, BIM, and PUMA activate BAXα by inducing conformational changes that expose the α1 helix, disengaging the α9 helix and freeing the dimerisation pocket (the BC groove). With the BC groove exposed BAX can interact with other BAX molecules forming dimers. The exact mechanisms and in what order they occur are still unknown due to the difficulty of studying BAX due its subcellular translocation and the dynamic interactions that occurs during apoptosis. The direct activators BIM, tBID and PUMA have been shown to interact with BAX at the α1 BC groove region, also nuclear magnetic resonance (NMR) has revealed that BIM interacts with BAX at an alternative binding site involving α1 and α6 residues (Gavathiotis et al., 2008). This suggests that BAX has two sites that it can be activated from. After the disengagement of the α9 helix, the direct activators remain associated with BAX through its BH1 domain. The direct activators then drive oligomerisation of BAX when inserted into the OMM.
Multiple isoforms of BAX exist. BAXα exists in the cytosol and is activated by conformational changes. Unlike BAXα, BAXβ (a primate specific isoform) exists in a constitutively active form where it spontaneously translocates to the OMM and undergoes oligomerisation resulting in MOMP. BAXβ initiates apoptosis in a BH3 activator-dependent manner (Fu et al., 2009). Baxα and BAXβ structurally differ only on their C terminal. As mentioned before the C terminal of BAXα regulates its activity by keeping it in an inactive form. The C terminal on BAXβ lacks the transmembrane domain (TM) and instead harbors a regulatory domain that couples it to the ubiquitin-proteasome system (UPS) for efficient degradation. BAX β is upregulated by inhibition of the UPS by apoptotic stimulation. BAXβ has also been shown to interact with and activate BAXα (Fu et al., 2009). BAXβ can also form heterodimers with BAXα demonstrating the plasticity of these effector proteins in their role in apoptosis.
BAK and BAX both initiate apoptosis through MOMP. BAX has slower killing kinetics than BAK during apoptosis due to its extra activation step in translocating to the OMM. Both BAX and BAK interact with BH3 direct activators through their own BH3 domains. These proteins have yet to be fully understood, and the conformational changes that occur during their activation and oligomerisation have yet to be fully elucidated. It is known that they play redundant roles in apoptosis but whether they interact with each other or merely increase the rate of MOMP when both activated is still unclear. It has been shown that under certain stimuli BAK and BAX can interact (Upreti et al., 2008) and it has been postulated that VDACs play a role in promoting BAX oligomerisation. Do the direct activator proteins drive oligomerisation in the OMM or are there other complexes aiding BAK and or BAX?
@RiskDebonair
Adventure Capitalist of the Future
This post has been ranked within the top 80 most undervalued posts in the second half of May 06. We estimate that this post is undervalued by $4.05 as compared to a scenario in which every voter had an equal say.
See the full rankings and details in The Daily Tribune: May 06 - Part II. You can also read about some of our methodology, data analysis and technical details in our initial post.
If you are the author and would prefer not to receive these comments, simply reply "Stop" to this comment.