BCL-2 homology and Signal transduction in relation to mitochondrial-dependent apoptosis, and neoplasia (part 1)
1.0 Introduction
Apoptosis serves as an important function in the human body, regulating cell death during development/differentiation, cell turnover throughout the body, and cell death due to cellular stress. During apoptosis the caspase proteases induce blebbing, cellular shrinkage, nuclear and chromosomal DNA fragmentation. This creates apoptotic bodies marked by phosphatidylserine on their cell surface, allowing them to be quickly and readily phagocytosed. In contrast necrosis (caused by acute cellular injury) leads to lysis of the cell membrane, releasing its intracellular contents which can damage surrounding cells and cause inflammation. Apoptosis confers an advantage over necrosis by effectively packaging and labelling the dying cells for clearance by the phagocytic cells.
Caspases are the initiators and executioners of apoptosis and are mainly activated by the mitochondrial pathway or the death receptor pathway of apoptosis. The intrinsic mitochondrial pathway is induced by intrinsic signals following cellular stress such as DNA damage, reactive oxygen species (ROS), hypoxia, growth factor deprivation, etc. The extrinsic death receptor pathway occurs via signal transduction when death ligands bind to death receptors.
Apoptosis is highly regulated, as deregulation can result in the pathogenesis of many diseases. Over expression of apoptosis can cause degenerative diseases, and under expression can result in diseases such as cancer. Understanding apoptosis is important as not only do the mechanisms involved cause carcinogenesis, but they can also be used and manipulated to target specific pathways with new drugs providing cancer treatments.
The B cell leukaemia/lymphoma 2 (BCL-2) family plays a key role in regulating the mitochondrial pathway of apoptosis by governing the permeabilisation of the outer mitochondrial matrix (OMM).When damaging cellular stress occurs, BCL-2 family members BCL-2-associated X protein (BAX) and BCL-2 antagonist killer 1 (BAK) oligomerise into proteolipid pores resulting in mitochondrial outer membrane permeabilisation (MOMP); this allows the efflux of apoptogenic factors such as cytochrome c and second mitochondria-derived activator of caspases (SMAC) from the mitochondrial intermembrane space. SMAC inhibits inhibitor of apoptosis proteins (IAPs). Once released into the cytosol, cytochrome c acts as a cofactor for the adaptor protein, apoptotic protease activating factor-1 (APAF-1). The binding of cytochrome c results in oligomerisation of APAF-1 which forms a disc-like complex called an apoptosome. Activator caspase-9 exists in an inactive form until it is proteolytically activated by the apoptosome, resulting in a cascade of further caspase activation (e.g., executioner caspase-3, and -7) causing proteolysis of intracellular proteins and cell death.
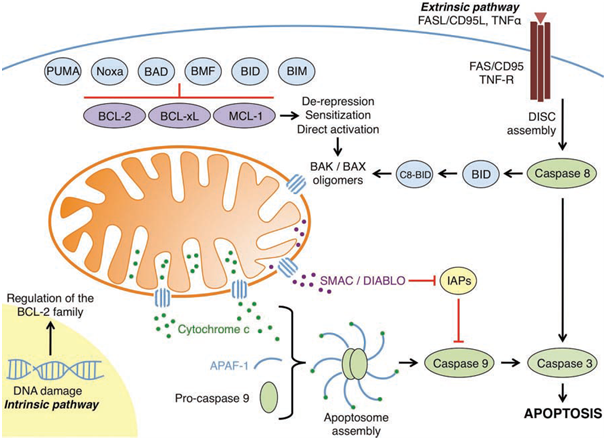
Figure 1: An overview of the intrinsic and extrinsic apoptotic pathways (Elkholi et al., 2011).
BCL-2 family members exert their control on the integrity of the OMM through a series of complex interactions that either results in survival, or commitment of the cell towards apoptosis. This crucial checkpoint at the mitochondria is regulated by a balance of pro-apoptotic and anti-apoptotic BCL-2 proteins, and their respective interactions. The BCL-2 family are characterised by the presence of at least one of the four BCL-2 homology (BH) domains (which mediate interactions between family members), and are functionally classified as either pro-apoptotic or anti-apoptotic, and can be divided into three groups.
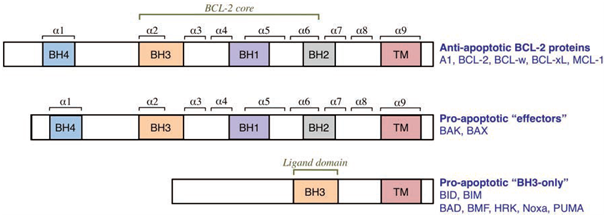
Figure 2: The three groups of the BCL-2 family (Elkholi et al., 2011).
The anti-apoptotic BCL-2 family members comprise of BCL-2, BCL-2-related gene, long isoform (BCL-xL), BCL-w, Myeloid cell leukaemia 1 (MCL-1), and BCL-2-related gene A1 (A1). These core members of the anti-apoptotic repertoire contain BH domains 1-4 and a C-terminal hydrophobic sequence that can direct them to the OMM. The anti-apoptotic proteins all share a similar 3D structure. They are generally found integrated in the OMM, but can also be integrated into the endoplasmic reticulum (ER) membrane or found in the cytosol. The anti-apoptotic BCL-2 family proteins directly inhibit the pro-apoptotic members (by binding and sequestering the BH3-only members) preventing MOMP and cytochrome c release. They can also bind to BAK and BAX, sequestering them and preventing oligomerisation.
The propapoptotic multi-domain (BH1, BH2, and BH3 containing, with a conserved BH4 motif) BCL-2 family members are effector proteins, BAX and BAK. BAX and BAK promote MOMP by forming proteolipid pores in the OMM when activated. BAK is found integrated into the OMM whereas BAX is mainly found in the cytosol and must translocate to the OMM when activated.
The other pro-apoptotic BCL-2 family members are known as the BH3-only proteins. The BH3-only proteins regulate the other two groups, and can be furthered divided into subgroups based on their interactions with the anti-apoptotic BCL-2 proteins and the pro-apoptoticBCL-2 proteins. According to the direct activation model there are the direct activators, BCL-2-interacting domain death agonist (BID), BCL-2-interacting mediator of cell death (BIM), and p53-upregulated modulator of apoptosis (PUMA) which interact with anti-apoptotic members and can directly activate BAX and or BAK, inducing oligomerisation and MOMP. BID is only active in its truncated form (tBID). The BH3-only proteins that interact only with the anti-apoptotic members are known as sensitizers and or depressors. A sensitizer binds to an anti-apoptotic protein (e.g., BCL-2-accociated death promoter (BAD) binding to BCL-2)leaving the direct activators free to activate BAX and or BAK; this lowers the threshold required for MOMP. The derepressors release effector proteins that have been sequestered by anti-apoptotic proteins (e.g., BCL-xL binds activated tBID. BAD then disrupts this interaction freeing tBID from BCL-xL, allowing tBID to then directly activate BAX resulting in oligomerisation and proteolipid formation). Some BH3 members act as both sensitizers and derepressors such as BAD. PUMA acts as a sensitizer, derepressor, and can also act as a direct activator highlighting the complex nature of these proteins.
@RiskDebonair
Adventure Capitalist of the Future
This post has been ranked within the top 50 most undervalued posts in the second half of May 04. We estimate that this post is undervalued by $4.45 as compared to a scenario in which every voter had an equal say.
See the full rankings and details in The Daily Tribune: May 04 - Part II. You can also read about some of our methodology, data analysis and technical details in our initial post.
If you are the author and would prefer not to receive these comments, simply reply "Stop" to this comment.