What is Deep Brain Stimulation (DBS) and How does it Work?
DBS might be a catchy medical term, but it is a very delicate procedure geared towards giving people with brain and nervous impairment, a new lease of life.
In 1997, the Food and Drug Administration (FDA) approved Deep brain Stimulation (DBS) essentially for cases and treatment of tremor. In April of 2003, DBS was also approved by the FDA as a treatment for dystonia.
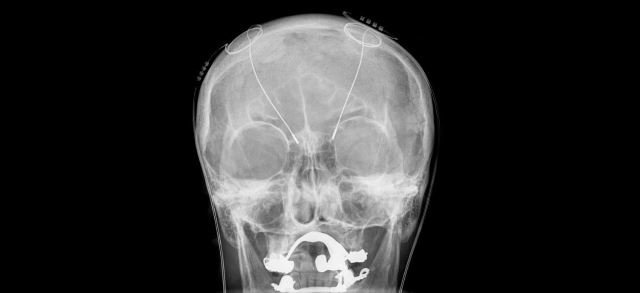
What exactly is Deep brain Stimulation?
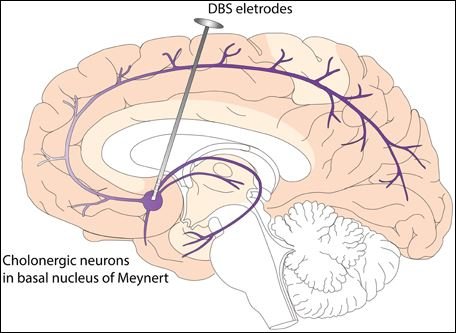
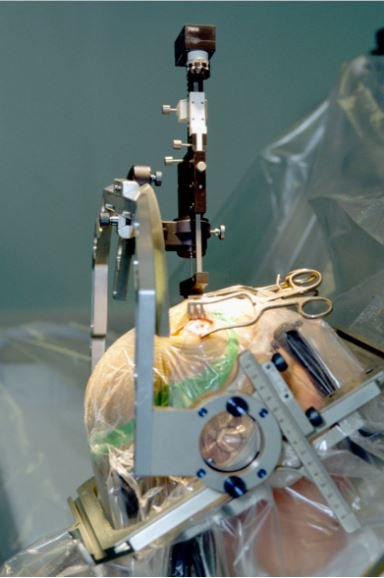
Deep brain stimulation (DBS) is a type of medical treatment and procedure which involves the surgical planting of a medical device known as a brain pacemaker, capable of sending electrical impulses to parts of the brain.
Back in 2002, the FDA approved DBS as a treatment for Parkinson’s disease. DBS is also capable of alleviating symptoms related to clinical depression which is often ‘treatment-resistant.’
However, despite the helpful nature of DBS, there are also some potential complications and side effects.
How does DBS work?
There are three major components of the deep brain stimulation procedure;
The implanted pulse generator (IPG)
The lead
The extension
The implanted pulse generator (IPG) is a neurosimulator which is battery-powered. It is enclosed in a titanium casing, and sends electrical pulses to the brain, in order to alter the neural activity at its target site.
The 'lead' of the DBS is implanted in one of the three areas of the brain. It is a coiled-wire insulated in polyurethane, with four electrodes which are platinum iridium.
The extension is an insulated wire running from the head of the patient to the downside of the neck and behind the ear, connects the “lead” to the IPG.
Optimum suppression of symptoms and control of side effects can be obtained through calibration by a nurse, technician or neurologist.
The 'leads' for Deep Brain Stimulation (DBS) operation is placed in the brain based on the type of symptoms it can address.
For instance, when treating the Parkinson’s tremors and essential tremors, the ‘lead’ is placed in the thalamus of the brain.
But for dystonia and related Parkinson’s diseases, the 'lead' is placed either in the subthalamic nucleus or globus pallidus
Beneficial application of the DBS
DBS treatment has been positive in patients. DBS has been shown to have the potential to reduce and eliminate epileptic seizures with certain stimulation.
DBS has also resulted in enhanced cooperation, euphoria and alertness, in persons with schizophrenia. Also persons with complex and partial seizures also experienced euphoria and sexual thoughts after a DBS procedure.
Via electric stimulation of the brain with electrodes, some persons also reported orgasmic ecstasy.
Side-effects of the DBS
Despite the remarkable benefits of ‘tweaking’ with the human brain, for ‘apparently’ medical and health purposes, there seems to be noticeable side-effects to the procedure of passing electrical impulses via electrodes to the brain.
Some of the obvious side-effects include; depression, euphoria, cognitive dysfunction, hypersexuality, apathy, hallucinations, etc.
These effects may be temporary and reversible if correct calibration of the stimulator and proper placement of electrodes is carried out.
All-in-all, I do believe the benefits of the DBS procedure has a massive potential for future harnessing of studies and science behind the brain’s neural activities, towards treating neuropsychiatric cases and persons.
Reference:
Thank you for your time and for reading my post.
If you found this post interesting, then kindly UPVOTE, RESTEEM and FOLLOW @rickie, for more quality posts.
You Can Check Out My Other Posts Below:
- Tree-Planting Drones – A Booming Business to Restore and Revive Earth
- Cranberry and Avocado Seed Husks Are Medically More Potent Than Previously Thought
- Apart From Humans, Pollution Affects Plants Extremely Too
- Treatment Of Ponds And Water Using Aquatic Plants
- Electronic Cigarette: History, How it Works, and Risks
- A Smart-Phone Compatible System Detects Bacteria In Food

Being A SteemStem Member
This will help our health! Thanks rickie. Can I resteem it?
Thanks for reading @htuan. And yes you can resteem it. And I don't believe you need any permission whatsoever to resteem my posts if you want to. Thanks again.
Why FDA? just wondering. Its like NAFDAC approving a surgical procedure in Nigeria.
Lolz....Yea, i see your point and analogy @gentleshaid.
But the DBS "brain pacemaker" chip or implant itself is classed as a "medical device", and so, under the United States's Medical regulations....
So, i guess that's why the approval of the FDA factored into the DBS procedure. Thanks for visiting @gentleshaid, I appreciate.
well explained. you are welcome :)