ICP-MS for Heavy Metal Detection in Supplements: Instrumentation
Instrumentation
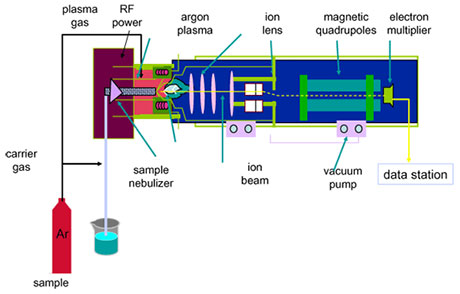
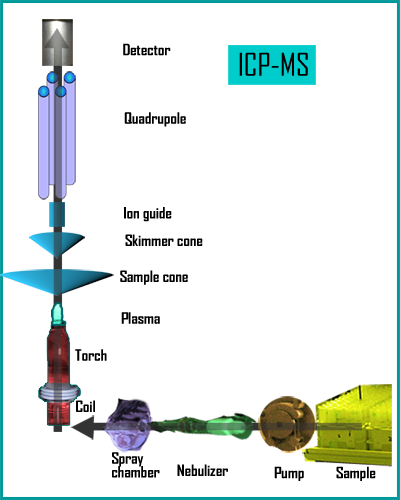
With inductively-coupled plasma mass spectrometry (lCP-MS), solid or liquid samples can be used. Solid samples are introduced into the ICP by laser abalation while aqueous samples are aspirated with high velocity argon, forming a fine mist in a nebulizer. Inside the spray chamber, larger droplets are removed so that only droplets small enough to be vaporized in the plasma torch make it through. In the torch body the sample is mixed with more gas and an RF coil is used is used to produce an argon plasma flame. At this point the sample is atomized and becomes ionized, while remaining solvent is removed (5).
The interface links the ion source at atmospheric pressure to the high vacuum mass spectrometer. Ions flow through an ~1 mm pore into a pumped vacuum system,and passed into the spectrometer at supersonic speeds. This is so that the ions can freely move without interference by air molecules, but requires a pumping system to maintain the vacuum. Next, a pumped extraction system produces an ion beam which is focused into the quadrupole mass filter. This consists of four parallel-aligned metal rods in a diamond pattern. Combined DC and AC potential are applied to the rods so that only a particular ion with a specific m/z ratio is able move through while others are deflected. lon detection is performed by means of an electron multiplier. When ions strike the dynode, a cascade of electrons is released which become amplified to a measurable level.
Analysis software provides quantitative and semi-quantitative data, as well as isotope dilution and ratio information. This is done by comparing the intensities of the measured pulses to those of standards, which have been used to obtain a calibration curve. Normally, only one isotope of an element is measured, because their natural abundances are fixed (4). However, lead is an exception to this, because naturally occurring lead can have two different origins. Some has been present since the formation of the planet while some has been formed due to radioactive decay, leading to variable lead isotope ratios. In this case isotope ratios must be summed to retain an accurate determination of lead
concentration.
If the sample to be analyzed is liquid, it must be broken into small droplets before introduction into the argon plasma (5). This may be by means of a peristaltic pump or through aspiration by a nebulizer. Several types of nebulizer exist that can be chosen based on viscosity and available sample volume to enhance introduction of certain sample types. DropIets are typically next passed though a spray chamber, which can be of several designs, including Scott and cyclonic chambers. These may be made of glass, quartz, or a polymer, and may be cooled or incorporate desolvation systems to allow the maximum number of small droplets into the torch while keeping out the larger ones.

What is this machine
To listen to the audio version of this article click on the play image.

Brought to you by @tts. If you find it useful please consider upvoting this reply.
Nice explanation dear explaining something in a blog article is hard @pinkspectre
@pinkspectra why a non-metal cannot be able to detect by such technique and how it is more useful compared to XRF which is also for metal analysis ?