Early Developments in Quantum Mechanics
Quantum mechanics, also called wave mechanics, was developed between 1900 and 1930 to solve certain problems that classical mechanics couldn't explain, such as blackbody radiation, and the photoelectric effect. Proceeding from basic observations provided by Max Planck, quantum mechanics has been very successful in explaining the behavior of atoms, molecules and nuclei. Planck's ideas were interpreted and developed mathematically by many giants of 20th century scientific development, including Bohr, Einstein and Heisenberg.
Max Planck, 1878. Wikimedia Commons
Blackbody refers to the electromagnetic radiation emitted by a heated object. At any temperature, a body emits electromagnetic energy in the form of thermal radiation, whose spectrum is a function of the temperature and physical properties of the object. A graph of experimental data for the distribution of energy in blackbody radiation shows that increases in temperature bring about increases in the total energy wile decreasing the wavelength of the peak distribution. In other words, the higher the temperature and energy, the shorter the wavelength of the radiation. This wavelength follows Wein's Displacement Law, where the peak wavelength times the absolute temperature is equal to 2.8977729(17)×10−3 m⋅K. However, classical theory did not match with experimental data. At longer wavelengths, the match is fine, but at short wavelengths, classical mechanics predicts infinite energy, while experimental results showed no energy at all! This contradiction is known as the ultraviolet catastrophe, and violates the law of conservation of energy. Planck aimed to solve this puzzle, by hypothesizing that the radiation was produced by sub-microscopic charged oscillations, called resonators. These resonators could only have discrete energies determined by the frequency of vibration and the quantum number n. This gave rise to ideas about quantized energy states, where energy is not increased or decreased on a spectrum, but on very specific "steps" of stability.
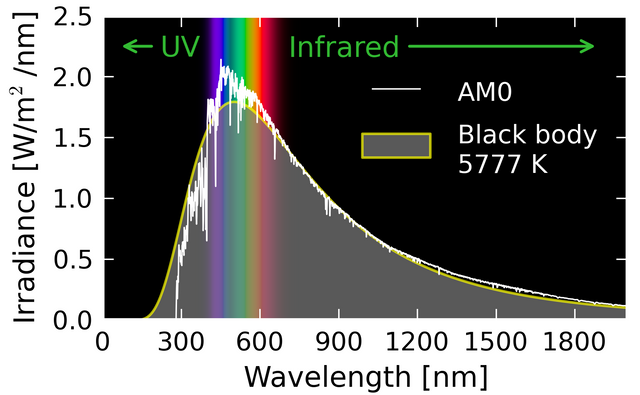 Blackbody Radiation, Danmichaelo. Wikimedia Commons
Blackbody Radiation, Danmichaelo. Wikimedia Commons
The photoelectric effect is the emission of electrons by an illuminated metal, first discovered by Heinrich Hertz in 1887. When light strikes certain metals, electrons, are emitted from the surface. The mechanism for the emission of these photoelectrons was debated until Einstein's successful explanation in 1905. These theories were a part of the paper he wrote on electromagnetic radiation for which he received a Nobel Prize in 1921. When light is incident on the metal surface, electrons are emitted so long as the frequency of the light is above a certain threshold, regardless of the intensity of the light. This threshold, known as the stopping potential, is determined by characteristics of the metal itself. Increasing the intensity of light at sub-threshold frequencies will not alter the lack of emission, but at frequencies above the stopping potential increased intensity results in increased kinetic energy of released electrons. Einstein extended Planck's idea of quantization to explain this phenomenon. He hypothesized that a beam of light is not only a wave propagating through space, but can also be interpreted as a collection of discrete particles (photons), each with energy hν. His insights into wave-particle duality served as validation for Planck's equation E = hν where energy (E) and frequency (ν) are affected by the quantization of energy. The factor h is Planck's constant or 6.626×10−34 J⋅s. When a quantized oscillator jumped from one energy level to the next lowest level, it would emit a photon, thus conserving the energy of the system. Einstein's theories were soon proven in experiments by Robert Millikan in 1914, in which he also validated E = hν and obtained an accurate value for Planck's constant.
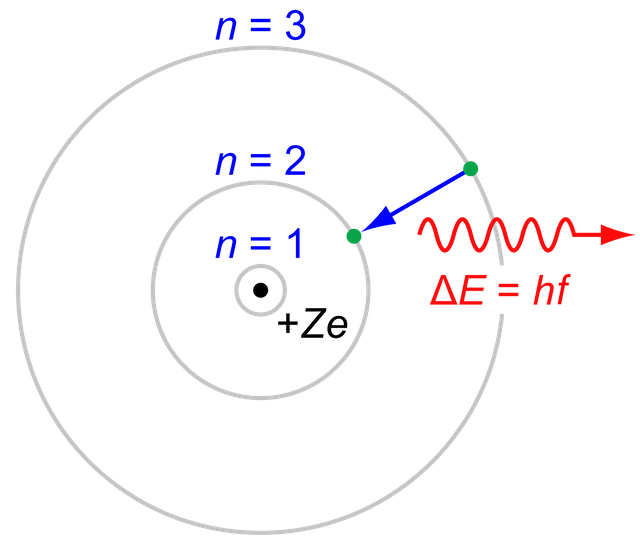 Photoelectric Effect, Jabberwok. Wikipedia
Photoelectric Effect, Jabberwok. Wikipedia
Source: 2nd Semester P-chem notes, Uva 2010.

Amazing informatic post. Keep sharing these types of informations to grow the knowledge of others. Good job! Keep it up.
Thank you!
Great post. It really shows what a revolutionary Max Planck was. I’m glad you gave a quick mention to Werner Heisenberg. I’m not an expert in quantum physics, but I’m a big fan of it in a broad sense. (The specifics can go over my head often) Germany really produced some exceptional physicists at that time.
Thank you! I'm no expert in any possible sense, but every time I have to explain it I understand a little more... I checked out your blog, great stuff. Will follow!
I just followed you too.
WARNING! The comment below by @shovon51 leads to a known phishing site that could steal your account.
Do not open links from users you do not trust. Do not provide your private keys to any third party websites.
To listen to the audio version of this article click on the play image.

Brought to you by @tts. If you find it useful please consider upvoting this reply.
Thanks for your sharing. It's convincing.
Fantastic story good job
Very Nice. Look here and I will provide you more votes http://mcg6000.com/booster