So What's The Deal With Graphene?
When science news articles about graphene get published, I generally notice two reactions from the readers’ comments: people get really excited, because apparently graphene is the most miraculous material ever discovered, and people get disappointed, because of the limited applications in which graphene has so far made the transition out of research and into production. What’s clear from both, however, is that the remarkable properties of graphene are poorly understood - it's true that it displays remarkable mechanical, electrical, optical, and thermal characteristics, but I think most popular scientific news articles tend to overstate what it can and will be used for. As my first dip into the Steem pool, I want to write a little bit and explore in somewhat simple terms what the hype is all about, and what the current state of research and manufacturing is.
Part 1: So What Is Graphene?
Graphene is a very special form of the element carbon, where atoms are arranged in a honeycomb pattern, a single layer thick:
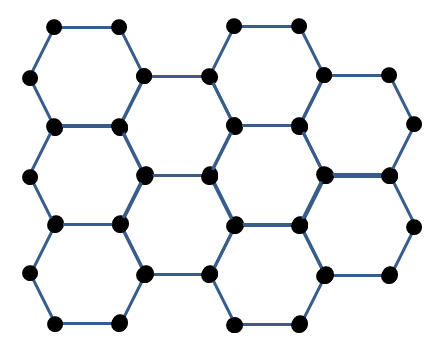
The dots in the image represent carbon atoms and the lines represent chemical bonds between them. It is this geometry and the characteristics of the chemical bonds that give rise to graphene’s unusual material properties.
Chemical bonds
A bit of the basics: every atom consists of a positively-charged nucleus, and negatively-charged electrons which surround it. The laws of quantum mechanics govern the geometry of where each electron is distributed around the nucleus. Those laws also dictate that the outermost electrons of atoms in close proximity can ‘pair up’ and form a chemical bond, or in other words a particular arrangement that draws the two nuclei together and holds them there. The distribution in space where each electron can typically be found is referred to as an ‘orbital’.
Graphene Orbitals
Every carbon atom has four valence electrons, ones that are free to form chemical bonds with neighbouring atoms. In graphene, three of these four form ‘sp2 hybridized orbitals’ which bond the atom in place with its neighbours in the characteristic honeycomb pattern. The final valence electron from each atom in a sheet of graphene remains ‘free’ to participate in what’s known as pi-bonding with the free electrons from neighbouring atoms. These pi-bonds are what give rise to graphene’s extraordinary electronic properties.
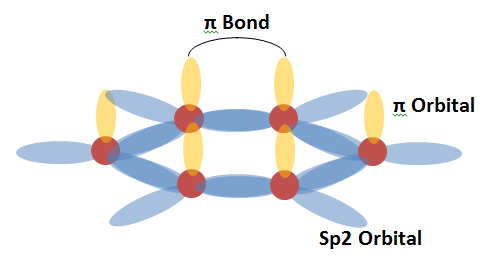
That's the very brief primer on what graphene is - a starting point for the origins of its material properties, and then onto some of the most recent developments.
Graphene is this super awesome, auto magic stuff. You should study it more.
Thanks for the encouraging comment complex! I've definitely got plans for so.e further articles on the subject - stay tuned.
Hi! This post has a Flesch-Kincaid grade level of 13.7 and reading ease of 41%. This puts the writing level on par with academic journals.
Congratulations @phaedrus! You have received a personal award!
Click on the badge to view your own Board of Honor on SteemitBoard.
For more information about this award, click here
Good!
Congratulations @phaedrus! You received a personal award!
You can view your badges on your Steem Board and compare to others on the Steem Ranking
Vote for @Steemitboard as a witness to get one more award and increased upvotes!