Energy Levels in an Atom (Atomic Physics Video Series – Part 3)
Where does the color of things come from? When white light shines on an object, shouldn’t the object reflect white light and therefore be white?
Your experience shows you that no... all things are not white!
This is because atoms (and molecules) absorb certain specific colors, while the others are just reflected. As a result, the object appears having the colors that are not absorbed…
The video presented in this post explains how atoms do that…
The first video of this series discusses the energy of an atom within the scope of the model defined by Niels Bohr. Such model describes an atom as being a nucleus around which electrons are in circular orbit. This is the model used for the whole series as an introduction to the amazing world of quantum mechanics.
The second video describes light as both being a localized wave and a particle, but most importantly, it explains that light is made of quanta of energy (fixed amounts of energy) called photons. The size of the quanta of energy of a given light defines the color of that light.
In the episode presented here, we will see that the energy of an atom can only exhibit certain values, in other words, the energy of an atom is quantized. This is why it is said that an atom has levels of energy. These specific energy levels can be calculated. We will explore this in detail in episode 4.
Here, in this episode, we will realize that an atom can gain or lose energy by absorbing or emitting a photon of energy corresponding to the difference between two levels. This is why a given substance will only absorb certain photons, and thus will appear as being of the complementary color to that of the photons absorbed.
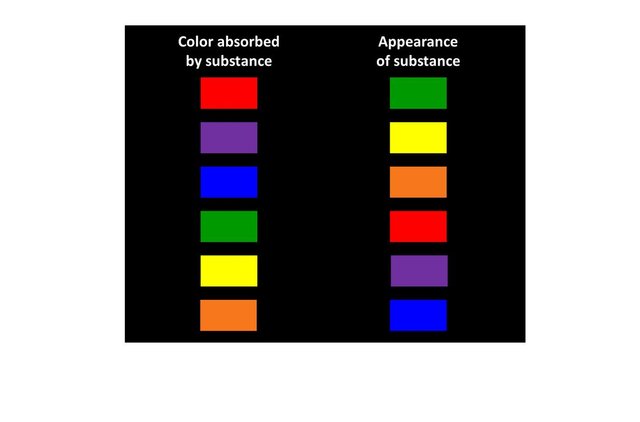
The table below depicts the color that would take a substance if its atoms only absorbed photons of a given energy, (i.e of a single color).
Of course, this simple illustration is just explanatory: an atom has more than two energy levels so it can absorb photons of various energies. It is the combination of the complementary colors of these photons that will define the final color of a substance.
Click on the video below to enjoy that fascinating voyage inside the atom.
This video series is aimed at providing an introduction to the quantum world to non-scientists and high school students preparing for their high school exams.
If you wish to deepen your understanding, please explore @lemouth quantum mechanics series. The relevant chapters to this specific video are:
Quantum mechanics lessons on Steemit - 5 - the mysteries of the atomic spectra
and
Quantum mechanics lessons on Steemit - 10 - the Bohr model of the atom
Hi,
I’m @muphy,
My life revolves around music production, teaching sciences, and discovery through travel.
You enjoyed that post? Resteem and Upvote!
You are interested in these topics? Follow me!

Sorry not to have found the time to watch the videos. I promise I will try (but I am super busy those days) :(
No worries! I know what busyness is. I just posted episode 4 today. And this even motivated me to set up the video studio again (no more living room ...) and then create an episode 5. The latter being much more scholar (solving exam problems), I still do not know If I'll post it on Steemit though...
I just read your article on the search of the 5th force at the LHC. Very cool :-). I finally understood how to read this mass/probability diagrams!
I finally saw it. I am really fan of your videos! For the next ones, tomorrow. Midnight here and I need to rest :)
I will probably look to all your videos in one shot during this week-end, when I will have some free time in Korea :)