Theory of Errors in Quantitative Analysis
As an analyst, we are required to achieve results as close as possible to true values, by applying the correct procedures. Whether it's during lab work or while doing research. But in fact, errors in an analysis are still common. An understanding of the theory of error in chemical analysis becomes very important. For that, in this article will be discussed Error in Quantitative Analysis and How to Reduce it.

Limitations of Quantitative Analysis Methods
Before we start further, we need to know the factors that influence the limitations of the analytical methods, namely:
- Accuracy
- Accuracy
- Source of error
- Chemistry involved in the analysis process
Accuracy (Accuracy)
Accuracy is the suitability between the measured results and the true value. There are two ways to determine accuracy by using absolute and comparative methods.
1). The Absolute Method
- Make a solution of pure substances that have known concentration (primary standard)
- Perform analysis with the procedure to be determined its accuracy, done several times, then averaged.
- Compare the results of the analysis with the actual content of the substance.
2). Comparative Method
Using comparative (not pure substances such as minerals) that have been established measure with methods that are considered more appropriate (standard method).
Accuracy (Precision)
Accuracy is the correspondence between the values of a series of measurements of a similar quantity. So for example we make a measurement, between the measurements with each other the results are the same or at least close together. Accuracy always accompanies accuracy, but high accuracy does not always mean "right".
The measure of precision is called the average deviation or coefficient of variation (KV). The formula for calculating accuracy:
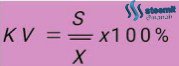
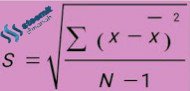
S: Standard deviation
X: The value of each observation
x bar: The average value of each observation
N: Number of data / Number of observations
A method is said to have good accuracy if KV is less than 3%.
Absolute (E) and Relative (E rail) Errors
Errors or commonly called errors will be widely encountered in quantitative analysis. Absolute error is the difference between the results of the analysis (x) and the actual price. While the relative error is the difference between the results of the analysis (x) with the actual price compared with the actual price.
Types of Errors in Quantitative Analysis
There are 2 basic types of errors in quantitative analysis:
A). Error assigned (determinate error)
Set errors are a mistake that can be avoided, the magnitude can be set, and occur repeatedly (one-way). The errors are divided into 4 sections:
Operational error
Errors caused by humans are self-transcending and have nothing to do with experimental methods or procedures.
Example:
- Non-quantitative treatment from analysts while experimenting
- Less precise quantitative sedimentation
- Heating / sediment heating at temperatures that are less precise
- Cooling the exchange rate is less sufficient before use weigh
- and others.
Error Instruments and Reagents
Error analysts when using instruments or choosing reagents.
Example:
- Instruments / tools used are not calibrated before use.
- The fatty / dirty burette is not cleaned before use
- The reagents used are not pure
- and others.
Method Error
Example:
- Incorrect sampling
- The reaction is not perfect
- The presence of impurities on deposits when using Gravimetry
- Selection of inappropriate indicators to determine the end point of the titration
Additive and Comparable Errors (Proportional)
The absolute value of the additive error does not depend on the quantity of the constituents of the substance set, whereas in proportional error it is very influential.
Example 1:
- The loss of the weight of the exchange rate at the time of the annealing will not affect the quantity of constituent substances placed in the exchange rate later.
Example 2:
- The presence of impurities in the standard solution causes the increase in the quantity of constituents linearly or not, so that it can result in an error of normality (N) value of a standard solution.
B). Error not set (accidental error)
Mistakes that occur even though the analyst has worked with the method of good and right with caution. For example, there is little difference in repeated measurements. This is caused by causes that can not be controlled by the analyst and generally difficult to understand.
How to Minimize Errors in Quantitative Analysis
There are several ways to minimize errors such as:
Calibrate tools and make corrections
The instrument is calibrated and corrected against standard measurements in order to determine whether the instrument used is in good condition (not damaged). In addition to calibration, it is also important to wash glass-sized appliances (eg biuret, volume pipette) to clean and impurities free.Setting the blanks
Make separate assignments to blanks. The objective is to know the presence of impurities in the reagents and the correction of the standard solution to reach the end point of the titration. Correction value should not be too big (not correct and not careful).Conducting surveillance
Under identical conditions, determination is made of samples and standards containing constituents of equal weight as contained in the sample.Using comparative analysis methods
Analysis using different methods, eg determination of iron content (Fe) in the sample by Gravimetry in comparison with the Volumetric method. The methods used are correct if the results obtained do not differ significantly.Perform a parallel assignment
Check the results obtained from the analysis and make repeated assignments. For example, titration is done 3 times not just once to get the right result. However, the titration volume obtained should not differ much or more than 0.05 (must be precision).
Meaningful Numbers
In addition to the above, to reduce errors in quantitative analysis is also very important to understand meaningful numbers. The number of digits is a digit that indicates quantity quantity. The quantity point here is all the "sure" numbers plus one "no" number for sure. The number 0 is a meaningful number, unless it is the first number of a number.
Example:
- 1.350 and 1.0024, zero here is a meaningful lift. So 1,350 and 1,0024 have 5 meaningful numbers.
Then how 0 is meaningless? Suppose 0.0035 kg, zero here is not a meaningful number because it only serves to determine the decimal place.
Rules For Calculation
a). Notice the meaningful numbers in each result so there is only one uncertain number.
Each device has different sensitivity, for example for balance analysis with sensitivity of 0.1 mg, weighing weight should be written four digits behind the comma (mg unit). Example: 2,3456 mg.
For other tools such as:
- 100 ml measuring flask should be 100.0 ml
- 10 ml volume pipes must be written 10.0 ml
- Burles with a scale of 0.1 volume should be written 2 numbers behind the comma
- Burles with a scale of 0.01 volumes must be written 3 digits behind the comma
b). Rounding
If the last digit is <5, then the number can be removed or unnecessary.
If the last number is> 5, then the number is rounded up.
If the last digit is = 5, then the number is rounded up if the number of the front is an even number.
Example:
4.75 can be rounded to 4.8
4.25 can be rounded to 4.2
c). Additions and subtractions
The end result has the decimal number according to the smallest decimal
Example:
152.12 + 5,034 + 0,5672 (the smallest decimal 2 digits), so it should be written 152.12 + 5.03 + 0,56 = 157,71
d). Multiplication and division
The number of significant numbers from the final result is equal to the number of significant numbers of the smallest data of meaningful numbers.
Thank you for reading my article on "Theory of Errors in Quantitative Analysis". I'm @manah. Hail the world of steemit.


Great post. Please state references to avoid plagiarism .
Thank you for your suggestion, i will do it in my next posting. but, i will do the source only on the image, is it allowed?
I highly suggest you supply all references to images and the article information. You would possibly see a better value of upvotes towards your posts, which is why you are posting these articles:)
I got a lot of knowledge from you about making my writing better. thanks. the suggestions from you are very useful, so my writing will get better in the next writing.
Thank you Master @zast🙏
👍😁
Great post. I'm Following. Please follow me...
Thank you for your comment, it's a passion for me. I follow you back
your welcome
@leeya got you a $8.89 @minnowbooster upgoat, nice! (Image: pixabay.com)
Want a boost? Click here to read more! @originalworks
The @OriginalWorks bot has determined this post by @manah to be original material and upvoted it!
To call @OriginalWorks, simply reply to any post with @originalworks or !originalworks in your message!
To enter this post into the daily RESTEEM contest, upvote this comment! The user with the most upvotes on their @OriginalWorks comment will win!
For more information, Click Here!
Special thanks to @reggaemuffin for being a supporter! Vote him as a witness to help make Steemit a better place!
Congratulations! This post has been upvoted from the communal account, @minnowsupport, by manah from the Minnow Support Project. It's a witness project run by aggroed, ausbitbank, teamsteem, theprophet0, someguy123, neoxian, followbtcnews/crimsonclad, and netuoso. The goal is to help Steemit grow by supporting Minnows and creating a social network. Please find us in the Peace, Abundance, and Liberty Network (PALnet) Discord Channel. It's a completely public and open space to all members of the Steemit community who voluntarily choose to be there.
This post has received a 0.52 % upvote from @drotto thanks to: @banjo.
Thank you @drotto and @banjo
You are welcome smiles back.
I am increasingly smiling because you reply to my comments. :D
Congratulations @manah! You have completed some achievement on Steemit and have been rewarded with new badge(s) :
Click on any badge to view your own Board of Honor on SteemitBoard.
For more information about SteemitBoard, click here
If you no longer want to receive notifications, reply to this comment with the word
STOPThis gem of a post was discovered by the OCD Team!
Reply to this comment if you accept, and are willing to let us promote your post!
If you accept this, you'll be nominated and the members of the OCD team will vote on whether we'll feature your post in our next compilation post.
You can follow @ocd – learn more about the project and see other Gems! We strive for transparency.
I'm mostly curating in #science and am always glad to see quality posts like yours peeking out between the garbage and plagiarism!
For the future, it might be a good idea to credit your pictures and link some sources so others can see your work is original and not plagiarized.
And if you want to connect with the science community on here, join us on steemit.chat in the #steemSTEM channel!
Thanks @suesa. I would be very grateful to @ocd who has chosen my writing for the share. it's a great honor for me.
https://steemit.com/factsaboutchina/@fouaz/7facts-about-china
This post made me laugh so much as I am into my dissertation and this post was the first one when I login into my account, when I just wanted to have a little break from quantitative research methods...Hahahaha :) Looking like I will not be able to run away from it in the next year :)