Biochemistry » Basic Concepts of Enzymes
Enzymes are biomolecules of proteins that act as catalysts (compounds that speed up the reaction process without reacting) in an organic chemical reaction. [1] [2] The initial molecule called the substrate will accelerate its conversion into another molecule called the product. The type of product to be produced depends on a condition / substance, called a promoter. All cell biological processes require enzymes to take place quite rapidly in a direction of metabolic pathway determined by the hormone as a promoter.

The enzyme works by reacting with the substrate molecule to produce the intermediate compound through an organic chemical reaction requiring lower activation energy, so the acceleration of a chemical reaction occurs because a chemical reaction with higher activation energy takes longer. As an example:
Y + XC → XYC (2)
XYC → CZ (3)
CZ → C + Z (4)
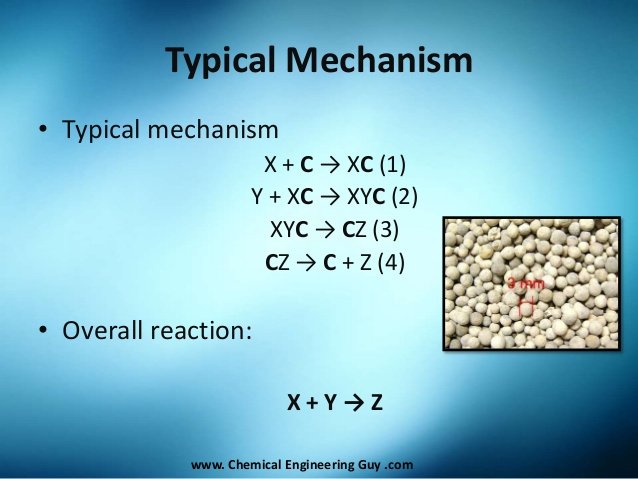
Although the catalyst compound may change in the initial reaction, in the final reaction the catalyst molecule will return to its original state.
Most enzymes work typically, which means each type of enzyme can only work on one kind of compound or chemical reaction. This is due to differences in the chemical structure of each enzyme that is fixed. For example, the α-amylase enzyme can only be used in the process of starch reshuffling to glucose.
The work of enzymes is influenced by several factors, mainly substrate, temperature, acidity, cofactor and inhibitor. Each enzyme requires different temperatures and pH (acidity levels) because different enzymes are proteins, which can change shape if temperature and acidity change. Outside the appropriate temperature or pH, the enzyme can not work optimally or its structure will be damaged. This will cause the enzyme to lose its function altogether. The action of enzymes is also influenced by other molecules. Inhibitors are molecules that decrease the activity of enzymes, while activators are those that increase the activity of enzymes. Many drugs and toxins are enzyme inhibitors.
Currently, enzymes have been widely used in various fields of application, especially the industry to assist the production process. This is because the performance of enzymes against susbtrat and catalyzed reaction types are very selective than inorganic catalysts.
The action of the enzyme can also be controlled by several effector to control the cell metabolism process. This allows the reaction in the cell to take place very efficiently.
The stability of enzyme molecules is strongly influenced by environmental factors, such as pH, temperature, and pressure. These factors can also alter the stability and three-dimensional structure of enzyme molecules.
Therefore, it is very important to understand the basic concepts of enzymes. In this article, we will discuss some basic concepts of enzyme which include: structure, function, excellence, substrate enzyme interaction and enzyme inhibition types.
Structure of Enzymes
Basically, enzyme molecules are proteins (one or more sub units), except ribozymes (RNA enzymes).
If an enzyme is composed of only protein molecules, it is called a simple enzyme. However, if composed of proteins and non-protein organic molecules then called the enzyme complex (holoenzyme).
Let's peel one by one of the components of this enzyme:
Apoenzymes are part of the protein of an enzyme that is not heat resistant and serves to determine the nature / specificity of an enzyme.
Cofactors are non-protein parts that help the activity of enzymes. Below are some types of cofactors, including:
1. Metal ions (Minerals) are non-protein parts of inorganic metal ions that act as activators.
Examples: Zn2 +, Mg2 +, Fe2 +, Ni2 +, and others.
2. Coenzymes are organic molecules of non-protein parts that are bound non-covalently in the enzyme. Coenzymes are synonymous with vitamins because many coenzymes come from vitamins and their derivatives.
Examples: NADH / NAD (Nicotinamide Adenine Dinucleotide), Coenzyme A, ATP (Adenosine Triphosphate), and others.
3. The Prosthetic group is a non-protein part composed of organic molecules (coenzymes) or metal ions that are strongly bonded covalently to the enzyme.
Examples: FAD (Flavin Adenine Dinucleotide), and others.
Enzyme Function
- As a biocatalyst in biological systems and determine the shape of chemical changes in cells.
- Increase reaction speed by decreasing activation free energy at transition state.
- Enzymes do not change the equilibrium of a reaction.
Enzyme Benefits As Biocatalyst
- High catalytic power
- High specificity, both of reaction type and catalyzed substrate.
- Can work on soft conditions, ie at temperature and pH are not extreme.
- The catalytic activity of some enzymes can be controlled.
- Can be produced, making it easier for availability.
Catalytic Processing by Enzymes
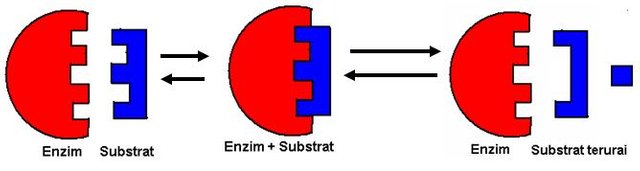
Enzymes bind to the substrate on the active side, will trigger the reaction and then release the product.
The enzyme's active side is the binding of the substrate and the prosthetic group, containing residues that directly play a role in the formation and breaking of chemical bonds. These residues are called catalytic clusters. The general description of the active side of the enzyme is as follows:
- The active side is a small part of the enzyme molecule, and blends with the three-dimensional structure of the enzyme.
- The active side of the enzyme lies hidden in a gap or pocket.
- The substrate is bound to the enzyme through various weak interactions in the enzyme complex (ES). The types of reactions involved include: electrostatic bonding, hydrogen bonding, van der waals bonding, and hydrophobic bonds.
- The specificity of substrate binding to enzymes is determined by the arrangement of atoms in the active site.
Substrate Enzyme Interaction Model
Enzymes can form a product if it binds to the substrate on the active side, The interaction model of the enzymes that are widely known there are 2, namely:
Key Models and Locks
The enzyme's active side has the same shape and size as the substrate.
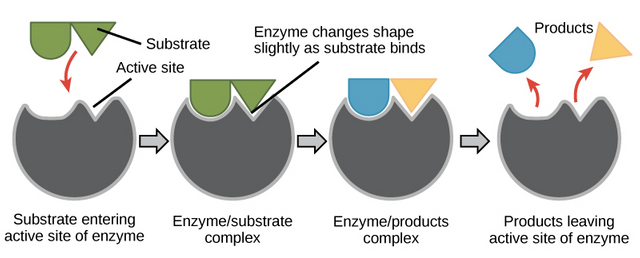
The Fit-induced Model
Enzymes and substrates undergo a deformation when bound to obtain conformations that stabilize the transition state.
Inhibition of Enzyme Reactions (Inhibition of Enzyme Work)
Enzyme performance can be inhibited by the presence of inhibitors. There are two types of inhibition by inhibition, namely:
1). irreversible inhibition
The inhibitor molecule tightly binds the covalently active enzyme side to permanently deactivate the enzyme.
Example:
DFP (Diisopropyl fluorophosphate) which binds the OH-serine group of the acetylcholinesterase enzyme (an important enzyme in the transfer of nerve impulses).
2). Reversible Inhibition
The inhibitor molecule binds to the non-covalent side of the enzyme's active enzyme, but can be detached again. Some types of inhibition can be reversed, namely:
Competitive Inhibition (competitive)
The shape and size of the inhibitor are the same as the substrate. In this inhibition, the substrate and inhibitor compete to bind to the active side of the enzyme. The affinity of the inhibitor to bind the active side of the enzyme is stronger than the substrate. However, this can be overcome by the addition of the substrate excessively.
Inhibition is not Competitive (uncompetitive)
The inhibitor only binds to the substrate enzyme complex outside the active site formed after the substrate is attached to the active site of the enzyme. Substrate binding modifies the structure of the enzyme, thus providing another binding side (allosteric side) to the inhibitor. The binding of the inhibitor on the allosteric side prevents the formation of the product.
Inhibition is not Competitive (non-Competitive)
The shape and size of the inhibitor is not the same as the substrate. The inhibitor binds the enzyme or substrate enzyme complex (ES) outside the active site. The binding of the substrate has not changed, but the substrate enzyme inhibitor complex (ESI) can not form the product.
Reference:
- https://www.slideshare.net/ChemicalEngineeringGuy/catalysis-and-catalytic-reactors-re10
- http://www.scienceclarified.com/El-Ex/Enzyme.html
- http://chemistry.tutorvista.com/physical-chemistry/enzymes.html


Holoenzyme
I'm sorry @justtryme90.
Thanks for fixing the mistake of words in my writing. it is very petrified me.
Thank you Master :)
You are welcome, but ... the Master thing is a little weird.
hahaha
What if I call as a friend?
Thank you friend @justtryme90
That works better for me :)
useful postings. develop. @originalworks
The @OriginalWorks bot has determined this post by @manah to be original material and upvoted it!
To call @OriginalWorks, simply reply to any post with @originalworks or !originalworks in your message!
To enter this post into the daily RESTEEM contest, upvote this comment! The user with the most upvotes on their @OriginalWorks comment will win!
For more information, Click Here!
Special thanks to @reggaemuffin for being a supporter! Vote him as a witness to help make Steemit a better place!
Congratulations! This post has been upvoted from the communal account, @minnowsupport, by manah from the Minnow Support Project. It's a witness project run by aggroed, ausbitbank, teamsteem, theprophet0, someguy123, neoxian, followbtcnews/crimsonclad, and netuoso. The goal is to help Steemit grow by supporting Minnows and creating a social network. Please find us in the Peace, Abundance, and Liberty Network (PALnet) Discord Channel. It's a completely public and open space to all members of the Steemit community who voluntarily choose to be there.
@minnowhelper
@minnowpond
@royrodgers has voted on behalf of @minnowpond. If you would like to recieve upvotes from minnowpond on all your posts, simply FOLLOW @minnowpond. To be Resteemed to 4k+ followers and upvoted heavier send 0.25SBD to @minnowpond with your posts url as the memo