Shed Some Light on Crime Scenes - Luminol
If you are a forensic science enthusiast and reader of detective stories, I got something for you. Probably you had watched series based on uncovering crimes like the CSI type. Especially as those who follow such programs know, when there is a possibility of murder but If the blood is not visible, after the area dimmed, researchers spray a liquid on the environment and in certain places and a bluish glow appears in some places. This bluish glow makes blood traces visible in the environment and thus the road to the uncovering of the murder begins. So far everything is normal. Well, if you would ask what does it have to do with chemistry. Get ready, here we go.

The bright bluish color that appears with spraying actually occurs as a result of a chemical reaction. Sprayed liquid is also luminol. So what is this luminol? How can a reaction help uncover the murders? Let's examine this.

Luminol (C8H7N3O2) is a compound that chemical named as 5-Amino-2,3-dihydro-1,4-phthalazinedione. Luminol was first synthesized in 1902. This molecule, the technical name 3-aminophthalhydrazide, received the name luminol at the end of 1920's. The use of luminol in judicial proceedings was recommended by scientists to use it for blood detection in judicial cases in 1942. Of course, the luminol, which makes this mysterious blue color, is not alone in the spray bottle. It is a solution with auxiliary components. If we take a look at the inside, a strong oxidizing agent must first be present in the bottle together with the luminol. This is usually hydrogen peroxide and is directly involved in the actual reaction. An another requirement is the basic solution. An alkaline base such as sodium hydroxide will work. This basic solution is necessary because luminol is a zwitterion in neutral solutions. So the molecule is both positively and negatively charged. But in the basic solution, luminol is in the form of an anion which is negatively charged, which can be oxidized by the oxidizing agents.
We learned the content of the solution but not everything is limited to that. There is a need for catalysis to accelerate the reaction, and the blood becomes a part of the activity right here. As we know, the hemoglobin that blood cells contain contains iron atoms. These iron atoms catalyze the reaction between luminol and hydrogen peroxide and help the reaction proceed. The peroxide formed during the reaction cycle rapidly turns into 3-aminophthalate. The resulting energy is transferred to the electrons of the 3-amino phthalate molecule, bringing them to a higher energy level. Electrons emit photons as they go down to low energy levels. This photon also gives the bright blue color.
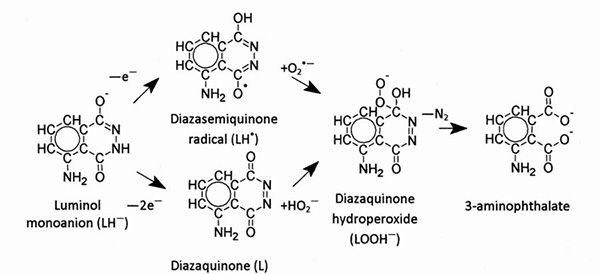
As in the case of luminol, a reaction is called chemiluminescence or chemical luminescence to light scattering. Energy in exothermic reactions as heat, in the case of chemiluminescence, it becomes open as light. The visible light, which as in our example, emits without heat, is called cold light. In fact, light scattering does not only occur in inanimate environments. Some creatures like fireflies also emit a similar light. This living thing is called bioluminescence.

Well, can we say that the blue glow that created with luminol always shows the presence of blood? No! Unfortunately, there are other substances that catalyze the oxidation of the luminol. Substances such as sodium chlorate in the bleach and low blood levels in the urine may elevate the luminol. Substances such as sodium chlorate in the bleach and low blood levels in the urine may oxidize the luminol. In addition, some enzymes can also help catalyze. Peroxidase, an enzyme found in the stool, can trigger the flame by chemiluminescence. An interesting item that can give a misleading result is wild radish. Of course, it is less likely to have a wild radish in the crime scene, but it is worth mentioning that it can result in misleading results. Even if the substance in the environment is really blood, it only can be confirmed with the tests.
There are other disadvantages of Luminol. The blue glow we see is much shorter than it appears in the series we watch. Approximately 30 seconds. In addition, using luminol causes damage to important markers such as proteins and enzymes in evidence, but it still appears that DNA samples can be taken for some uses. In addition, a water based solution can dilute blood and cause it to disperse. I mean, although it is indispensable in the series like CSI, it is not a substance that is used more often than it actually appears in real life. But we still know it's useful.

One important element in the crime scene that helps detect criminals is fingerprint. Fingerprints are very important because they are personspecific. But fingerprints are not always visible and smooth. Investigators often use chemical methods to reveal fingerprints. Let's look at the classification of fingerprints before methods. Patent, plastic and latent fingerprints are divided into 3 pieces.
Patented fingerprints are visible. These fingerprints are visible with blood or ink. The fingerprints called plastic are similar. It can be seen with the naked eye. They can be seen on a flexible material such as clay or wax. Since these two are visible, they can be recorded directly with the camera.
The problem is latent fingerprints. These are traces left by the oil and sweat that the body naturally creates. Despite being in the touched area, they can not be seen without an extra intervention. Here is where the chemistry comes into play.

One of the methods used for detecting fingerprints is powdering of the fingerprints. Its use dates back to 1891. This method is very useful for looking for a fingerprint that will not be absorbent, even if it is going to be done in 4-5 hours. These powders are characterized by fine grains and a heavy structure. This fine powder is spread on the surface with the help of a brush. These powders help to make fingerprints visible by holding onto materials such as sweat and oil that form latent fingerprints. The visible fingerprint is photographed or taken from the spot detected with a band.
Fingerprints are different from each other. Let's examine the properties and uses of these powders.
Ceruse
It is a heavy white powder and is used to indicate invisible fingerprints because it is very thin.
Graphite Powder
It is black in color and is used successfully on white surfaces, especially on paper.
Antiuman Sulfur
It is black in color and heavier than graphite.
Aluminum Powder
It is mostly used because it has a light structure on the surfaces like porcelain.
Mercury Sulfur
It has a heavy structure and is red in color.
Lead Sulfur
It is white in color and used in like white paper.
The point to note in this method is the fingerprint of the material to be used should be in contrast to the color of the powder to be used and also this method is not perfect. Although the powders are carefully spread with a fine brush can damage to fingerprints. Another problem with the brush is the transfer of DNA. For this reason, researchers apply different methods.
Another method of ensuring that fingerprints are visible is accidentally discovered. When scientists worked on cyanoacrylates, which are molecules used in superblockers, they noticed that the fingerprints on the instrument they were using became visible with the smell of cyanoacrylate in 1982. This has shown that the cyanoacrylate is polymerized in contact with fingerprint residues. This method is used exclusively on smooth surfaces. The time required for the cyanoacrylates to concentrate on the fingerprint is short. It takes even shorter when heated to evaporate. This is advantageous. Moreover, the fingerprint detected by this method is resistant and the sooner the fingerprint is left the more effective the method is.
.jpg)
Too many chemicals can be used to make invisible fingerprints visible. Some of these make the fingerprint colorful, while others cause it to shine under certain lights. One of the known chemicals is ninhydrin. Ninhydrin was discovered by Siegfried Ruhemann in 1910. Siegfried Ruhemann discovered that this material was transformed into the purple with the skin. This purple color is now referred to by his name. Ruhemann's purple. Although it is known that it will help to reveal invisible fingerprints, the use of this time from 1954 is not often seen.
Sweat contains amino acids (250 nanograms per fingerprint). Ninhydrin reacts with these amino acids when sprayed to the surface, revealing the ammonium salt of Ruhemann's purple and a clear purple color.
The water is an important reactant for this reaction and should therefore occur under high humidity conditions. However, The Ruhemann's purple is decomposed in the presence of light and oxygen. For this reason, fingerprints should be kept well and photographed.
As an example of other chemicals that show similar properties of ninhydrine, we can say 1,8-Diazafluoren-9-one (DFO) or 1,2-indanedion. DFO resembles ninhydrin by reacting with the amino acids of the finger. A pinkish red color appears in the center and shines when illuminated by the blue-green light.
Congratulations @kedi! You have completed some achievement on Steemit and have been rewarded with new badge(s) :
Click on any badge to view your own Board of Honor on SteemitBoard.
For more information about SteemitBoard, click here
If you no longer want to receive notifications, reply to this comment with the word
STOPThis post has received a 3.85 % upvote from @buildawhale thanks to: @kedi. Send at least 1 SBD to @buildawhale with a post link in the memo field for a portion of the next vote.
To support our daily curation initiative, please vote on my owner, @themarkymark, as a Steem Witness
img credz: pixabay.com
Nice, you got a 75.0% @minnowbooster upgoat, thanks to @kedi
It consists of $16.85 vote and $5.62 curation
Want a boost? Minnowbooster's got your back!
The @OriginalWorks bot has determined this post by @kedi to be original material and upvoted(1.5%) it!
To call @OriginalWorks, simply reply to any post with @originalworks or !originalworks in your message!