Chemistry Express: Ions and Ionization Energy

Anions and cations
Atoms can have an overall positive charge, a negative charge, or no charge at all. In a neutral atom, the amount of positively charged protons equals the number of negatively charged electrons. This means that their charges cancel out leading to a zero net charge (no overall charge).
There are cases however, in which an atom loses or gains one or more electrons. This causes the net charge of the atom to shift to negative when it ends up having more electrons than protons, and to positive when it ends up having more protons than electrons. These charged atoms are called ions.
An atom that has gained extra electrons is called an anion. Since electrons carry a negative charge, the overall charge of the atom is now negative (-). On the other hand, an atom who has lost electrons is called a cation. Since the atom now has less electrons than protons, its net charge is positive (+).
Some people confuse the two notions because they tend to associate “losing” electrons to the minus sign. Remember that the minus sign does not represent the effect of losing, but the final net charge of the atom!
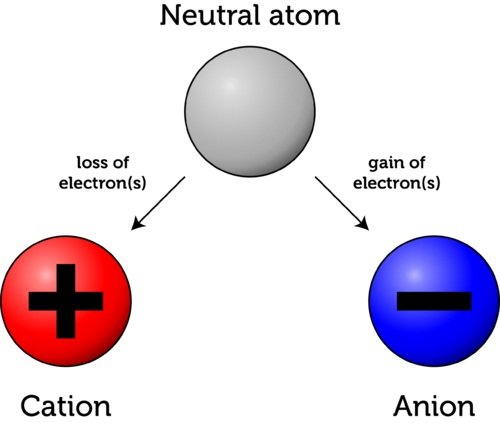
image credit
For example, if we remove one electron from hydrogen (H), it gets ionized and turns into a cation:
H+1 + e-1, or H+ + e- for simplicity.
If we add an electron to chlorine, it turns into an anion:

image credit
Ionization energy
Ionization energy is the amount of energy required to remove the most loosely bound electron of an atom. This depends on the atoms of each element, on how many valence electrons they have, as well as how many electron shells they have.
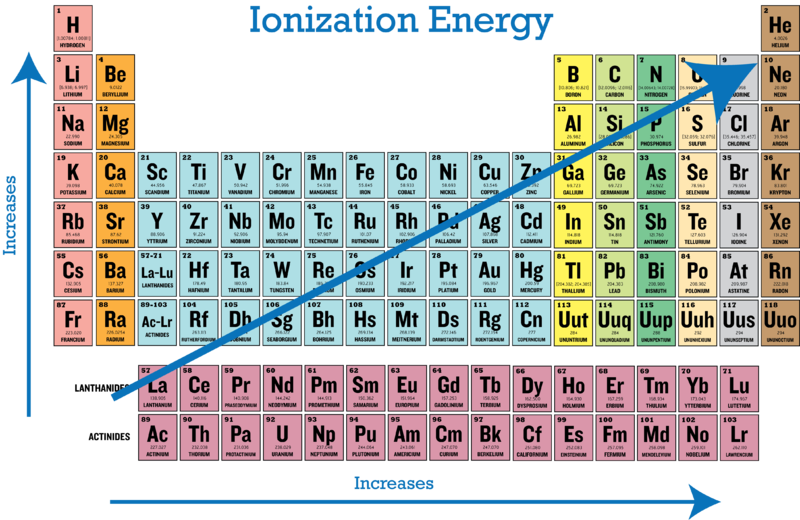
On the periodic table, the first group of alcali metals have only one valence electron each, which they are willing to give away in exchange for valence shell stability. Therefore, very little energy is required to cat-ionize them. On the other end, the noble gases are so stable that a very high ionization energy is required to remove one of their electrons and destabilize them. We realize that there is a trend on the periodic table: the ionization energy required to remove an atom’s electron depends on the number of valence electrons and thus increases from left to right.
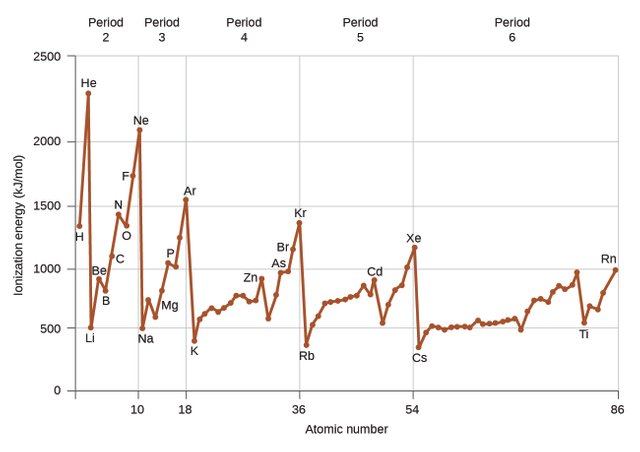
image credit
Horizontally on the periodic table, the first period comprises of small atoms with few protons and electrons. As we move downwards, more electron shells are added to the atoms of each period. The more electrons an atom has, the weaker is the attraction to the nucleus. This happens because of the electron shielding effect. Basically, the more electrons there are in an atom, the more they repel each other, because they are all negatively charged. Electrons from inner shells repel the ones from outer shells. This causes the outermost electrons to be pushed far away and the overall atom size to increase. As a result, the outermost electrons are less attracted by the nucleus and easier to remove.
Therefore, the ionization energy required to remove an electron is higher on the first periods and lower as we move to the bottom. It's important to note that for the same reason, atoms that receive extra electrons and become anions increase in size, while atoms that give away electrons and become cations, decrease in size.
Combined, these observations lead to a diagonal trend: The lowest ionization energy is required for atoms on the bottom left, and the highest for atoms on the top right.
.jpeg)
image credit
Ionization energy is measured in electron volts, which in turn can be converted to joules. Helium has the highest ionization energy of all elements, with 24.6 electron volts and francium has the lowest, with 3.8 electron volts.

Science is cool!
Yes it is!
Great work my friend @elemaya, we improve science and chemistry here, to share experience each other
Thank you, @jamhuery! Thank you for the resteem as well :)
Cool post, I am super into ionization, alkaline/acidic ratios and negatively charged particles for health reasons.
This is important information very few people actually know haha.
Thank you :)
SteemON!
Congratulations @elemenya! You have completed some achievement on Steemit and have been rewarded with new badge(s) :
Click on any badge to view your own Board of Honor on SteemitBoard.
For more information about SteemitBoard, click here
If you no longer want to receive notifications, reply to this comment with the word
STOPIf you want to see some ionization in action, check out the first picture of my post here: https://steemit.com/photography/@roblux/this-is-what-happens-if-you-aim-your-dslr-for-2-5h-at-the-night-sky
It's a cloud of glowing ionized gas!
Very interesting post. I followed!
Wow! Nice to see something related to studies here.
Very informative post about cations and anions. Ionization energy depends on the no. of valence shell and valence electrons. This is greatly explained through the Periodic Table.
Thanks @elemenya for bringing awesome study content to steemit. :)
Thank you @nitesh9. I'm trying to do my best :)
Great work. Wishing for your great success here. :)
Excellent material. My teenage daughter is doing GCSE Chemistry at school so I will show this to her. Hopefully she can follow along with your lessons here.
Thank you. I hope my posts can help her with her studies! Chemistry is a wonderful subject and its really not that difficult if you grasp the basic intuition about how it works :)
I will pass that on to her.
More science on Steemit, the better :-)
Thank you :)
It would help if this series had a special tag so I could get a list. Like maybe chemistryxpress?
It's a great idea, thank you for the suggestion!
well-described "mechanics" of the process...but why Ionization is important, what's it doing? And, yes my chemistry teacher didn't infuse me with much of inspiration for the topic, that's why asking :))