The King of Metals (Part 2): Simple gold Mining Methods [English version]
In the previous article; King of Metals (Part 1), we talked about the gold, its chemical and physical properties, its abundance in nature and places to find it. Where we said that gold is not available in nature in its pure state, for this reason, some methods must be used to extract and purify the gold.
Gold comes in the form of small granules scattered in certain types of rocks or in the sand, where it is very rare to find a large piece of gold.
The first step is the extraction of gold: there is more than one way, but the gold extracted is not pure and is often mixed with other metals such as copper or silver. So we go to the second step, purification, where we separate gold from other metals based on the physical properties that vary from one metal to the other to get 99.99% pure gold.
Some extraction methods:
1)The amalgamation:
This method is used to dissolve gold in mercury, because gold is soluble in mercury, although amalgamation is considered somewhat conventional and internationally prohibited, this method is used to extract small gold particles from some amateur miners and some mines in third world countries.

Une mine d'or par méthode d'amalgamation datant du 19ème siècle, taken by Karl Gruber; source: wikipedia
This method begins with a mechanical treatment, where the ore is broken into small pieces that can be crushed and cut into a special mill called "amalgam banel".
After grinding, we obtain a fine powder.
The powder is placed in a container with mercury-containing water, the mercury reacts with gold (and the gold-bound metals) and forms a solid mass that remains at the bottom, while the stone granules are dissolve with water.
The final step is heat treatment at 600 ° C, where the mercury mixed with gold is placed in a heat source and after a period of time, the mercury evaporates and there is gold remaining (70%). This is the most dangerous step in this process because the inhalation of mercury directly attacks the nervous system.
- "Mercury extracts gold and reduces age"!
Amalgamation is used in many developing countries because of its ease. As the price of gold has increased, many miners have begun to try their luck, for example; this method is widespread in Sudan and many other African and Asian countries. Many still use them despite the danger of toxic mercury vapor, resulting in very serious health risks, in addition, the environmental pollution caused by this process, which negatively affects the atmosphere.
According to the United Nations Environment Program (UNEP), gold mining using mercury accounts for 37% of anthropogenic mercury emissions. According to the International Labor Organization (ILO), nearly one million children work in gold mines in Africa, Asia and Latin America, although human rights and health organizations demand that governments improve working conditions.
2)Cyanation:

Pixabay
Gold is extracted with sodium cyanide (NaCN) or potassium cyanide (KCN) at a concentration of 0.05%. This method is the most used and was introduced in 1890 by "MacArthur" and "Forrest" when using potassium cyanide to melt gold. This process is used when the gold concentration is high in the ore (more than 20 g of gold per tonne).
At the beginning, the ores are crushed and placed in large tanks to be treated in the air where sufficient quantities of oxygen must be present by adding a solution of potassium cyanide and distilled water, the process is carried out according to the following chemical formula:
2Au+ 4KCN+ H2O+ O======================= 2[AuK(CN2)]+ 2KOH.
After the reaction of the cyanide solution with gold granules, the compound [AuK (CN2)] is formed, this process is done to separate the gold from the ore powder. This compound is then treated with zinc to separate the gold from cyanide according to the following chemical formula:
2[AuK(CN2)]+ Zn========================= 2Au+ ZnK2 CN2.
3)The borax method:
Extraction of gold with borax (Na2B4O7.10H2O); this method is frequently used by miners on a small scale, which distinguishes this method is that it is cheap and relatively safe compared to the methods mentioned above. Where borax is not considered harmful to the environment because of its absence of toxic substances.
Properties of borax, it facilitates the melting of metals (the melting point of metals decreases when borax is used), and able to fade gold, so you always need to add ammonia to get gold in its natural color after the operation.
Borax is used in place of the mercury going through the same steps and the gold extracted with the use of borax is purer than mercury-extracted gold.
Purification:
Gold extracted from previous operations is not clear and contains impurities, such as iron or copper, to separate gold from impurities. We use the method of electrolysis or heating method depending on the difference of the melting points of each metal.
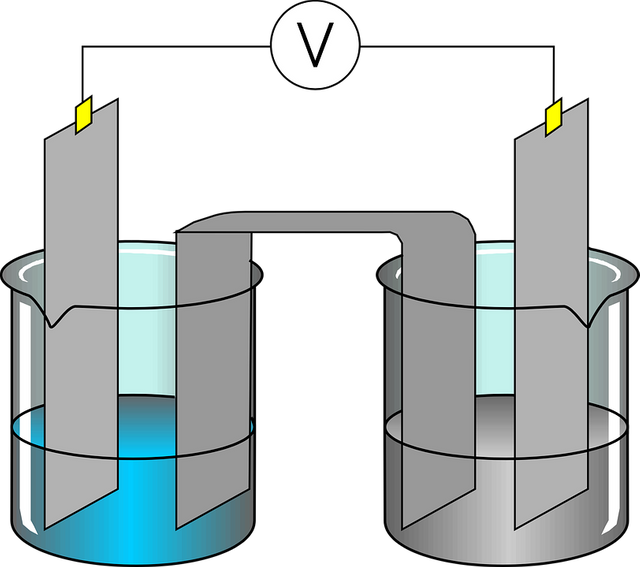
Pixabay
% Gold content in certain ores:
Hessite (Ag3 Te): 5%.
Krennerite (Au Ag Te10): from 31 to 44%.
Electrum (Ag): 45 to 75%.
- The largest gold coin was found in 1869 in Australia weighed about 71 kg.

Source: Wikipedia, (CC BY-SA 3.0)
Références:
Or, Wikipedia
Gold processing
Revue de physique et de chimie et de leurs applications industrielles
Extraction de l'or, nouvelles solutions et methodes plus ecologiques
Congratulations @benainouna! You have completed the following achievement on Steemit and have been rewarded with new badge(s) :
Click on the badge to view your Board of Honor.
If you no longer want to receive notifications, reply to this comment with the word
STOPDo not miss the last post from @steemitboard:
SteemitBoard and the Veterans on Steemit - The First Community Badge.