You are viewing a single comment's thread from:
RE: Salt and Sugar Under The Microscope 🔬
Thank you for this informative article. I would like to add some points to readers about the difference between salt and sugar in chemistry:
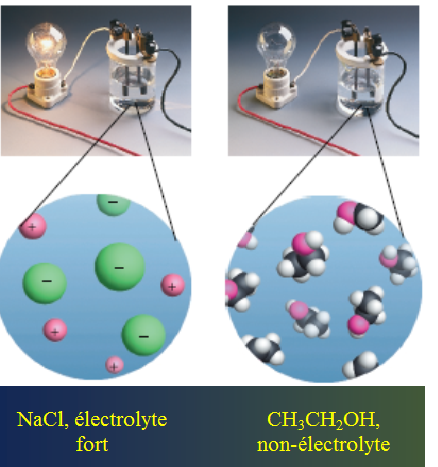
When the salt is dissolved in the water, an ionic solution is formed with two types of electrolytes: positive electrolytes(+) and negative electrolytes(-). and in this solution the number of negative electrolytes is equal to the number of positive electrolytes which means that the total charge of the solution is zero.
- The cation Na+ Indicates that the sodium atom has lost one electron:
Na======== (Na+) + 1e- - The anion Cl- indicates that the chlorine atom earn one electron:
Cl + 1e- ======= Cl-
As a result of positive + and negative - charge moving in two opposite directions within the solution, this solution conduct electrical current. Unlike molecular solutions such as water solution + sugar, the electric current can not be transmitted because there are no ions in the solution.
Have a nice day, keep on writing good articles, i wish you a good luck
That's a very nice comment and it complements the article. Networking is the best way to make friends and attract attention to yourself while at the same time being considerate to fellow writers.
Thank you very much :)