Pop Chemistry #1: Salt in our life
Salts are more than just “table salt”…
If we talk about “salt”, the first thing that comes out from your mind will be “table salt”. As a chemist I call it sodium chloride (NaCl). Actually “salt” can be found everywhere in our daily life. Two common examples are baking soda (sodium bicarbonate, NaHCO3) and marble (calcium carbonate, CaCO3). In this article i want to introduce to you what "salts" actually are and why they are necessary in our life.

So… what is “salt” in chemistry?
In Chemistry, a salt is defined as an “ionic compound” that consists of a “cation” (positively changed ion) and an “anion” (negatively charged ion). They tend to come together and form a “bond” between them… It is like a boy and girl who are attracted to each other!
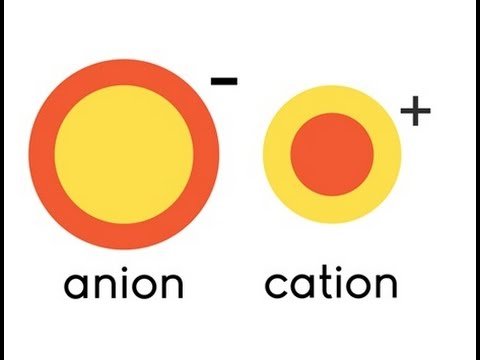
There are variety of “salts” in the Chemistry world and here i am not going to highlight them one by one. We sometimes classify the “salt” based on how well they can be dissolved in water. Most of them such as sodium chloride (table salt) and potassium chloride (electrolyte replenisher) readily dissolves in water while some of them does not dissolve at all! The solubility of the “salt” depends on the strength of the bond between the cation and anion.
Why are salts special?
Salts are stable and their properties remain (mostly) unchanged under ambient conditions if stay alone. However, if you put “salt” together with other substances, it may generate unpredictable “chemical” changes!
For instance, people use vinegar to remove salt buildup in pipes that slow down the water flow. The major culprits of the clogging are magnesium carbonate (MgCO3) and calcium carbonate (CaCO3). These salts belong to the carbonate family and are sparingly soluble in water. So why vinegar can be used to remove these salts? Actually, one major component in vinegar is called acetic acid (CH3COOH), it can react with the carbonate salts and convert them into other highly soluble salts and generate water and carbon dioxide gas as side products.
Below are the chemical formulae behind this phenomenon:
MgCO3(s) + 2CH3COOH(aq) ------> Mg(CH3COO)2(aq) + H2O(l) + CO2(g)
CaCO3(s) + 2CH3COOH(aq) ------> Ca(CH3COO)2(aq) + H2O(l) + CO2(g)

Another related application would be to use baking soda (sodium bicarbonate, NaHCO3) and vinegar (acetic acid, CH3COOH) to clean up your drain. The carbon dioxide gas generated from this reaction helps 1) break down the soap and scum that adhere on the wall and 2) shove the clog down.
Below is the chemical formula behind this phenomenon:
NaHCO3(s) + CH3COOH(aq) → CO2(g) + H2O(l) + CH3COONa (aq)

Let’s talk about some nitty-gritty
If you think "table salt" is more than just a seasoning, you are completely wrong! Sodium chloride is an indispensable component in our body. The primary function of sodium chloride (or more precisely their corresponding ions) is to help regulate blood pressure and control fluid balance. Another important aspect of these electrolytes is they participate in many physical/chemical reactions that maintain the functioning of our body.
Actually Sodium (Na+) and potassium (K+) ions can directly influence our brain functions! How? We have to talk about the nerve cells (also called neurons). These are special cells in your brain that serve as information carrier. The nerve cells are not directly connected to each other. There is a specialized “gap” between two nerve cells called synapse. The synapse is responsible for passing on signal from the “deliver” cell (presynaptic neuron) to the “receiver” cell (postsynaptic neuron). By manipulating the direction of the ion flux, the receiver cell can receive the information transmitted from the “deliver cell” and pass along the neuronal network. If you are interested in the details I highly recommend you to watch these amazing YouTube videos: h ttps://www.youtube.com/watch?v=OZG8M_ldA1M and h ttps://www.youtube.com/watch?v=VitFvNvRIIY
Besides sodium and potassium ions, other “cations” are also important in various biological phenomena. For example, calcium ion (Ca2+) is a necessary component in muscle contraction, iron ion is an essential element for blood production and is responsible for oxygen transport etc.
I hope by the time you finished this article you will know “salts” not just simply refer to “table salt” and have a lot to do both inside and outside of your body.
If you like what I write. Please follow me and give me an upvote ;)
About me:
I am a senior graduate student working in the field of biomolecular chemistry. Specifically I research on how changes in physical environment influence the downstream biochemical signaling of cells. I discovered steem recently and start blogging on this platform.
Nice post, i followed your account, please follow me at @mrrandy
Congratulations @alchemist89! You have received a personal award!
Click on the badge to view your Board of Honor.
Do not miss the last post from @steemitboard:
SteemitBoard and the Veterans on Steemit - The First Community Badge.