Storing energy in thin air?
No quite, but kind of.
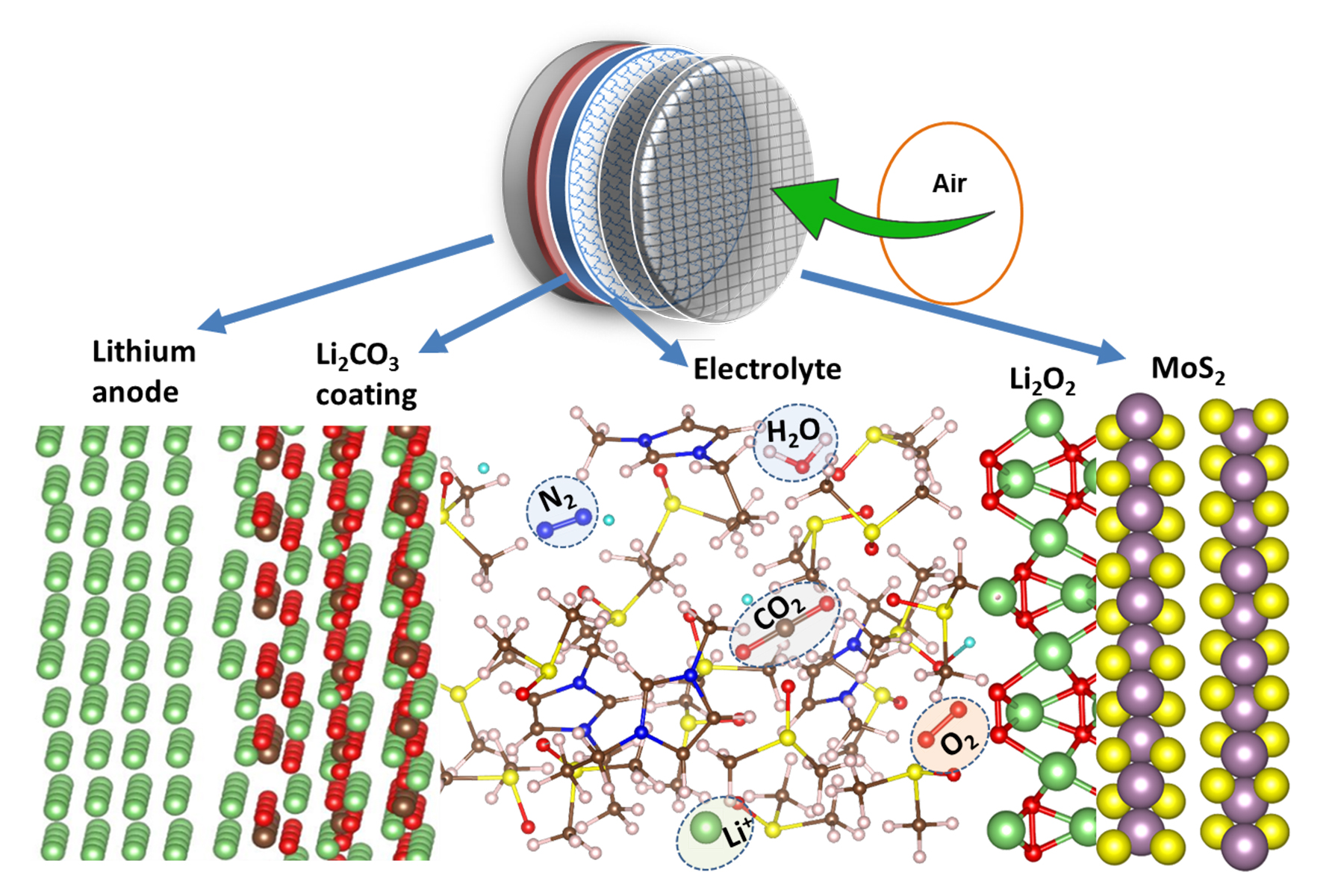
Lithium air energy storage uses lithium and combines it with oxygen in the air to form lithium peroxide, and back again.
So when the battery is discharging, oxygen and Lithium reacts to form Lithium peroxide, and then when the battery recharges, this is broken back down into lithium and oxygen.
Using air as one of the two reactant material means the battery saves on space and weight which could make Lithium air batteries 5 to 10 times more energy dense than the existing Lithium Ion batteries available today.
The resulting impact on applications like electric vehicles, drones & mobile electronics could be huge. And the technology could even lead to advancements in battery powered air transportation.
Of course there are still tech development challenges but recent advancements by the University of Illinois at Chicago are showing signs for optimism.
To listen to the audio version of this article click on the play image.

Brought to you by @tts. If you find it useful please consider upvote this reply.
While this tech does make some improvements, we are far from realizing true lithium air batteries. Some of the issues are (not a comprehensive list):
Filtering out CO2 and H2O while allowing O2 (if assuming open to the outside air system)
Carrying a dangerous and heavy O2 tank (if assuming a closed system)
Lowering the overpotential by electrocatalysts
Improving the coulombic efficiency of whatever electrolyte is chosen for round trip efficiency
Improving C rate
Ensuring that the packaging that is chosen does not nullify the energy density benefits when compared to lithium ion
Proving the safety record (nail test, short circuit test, vibration test, etc. and corresponding certifications).
I don't mean to dampen enthusiasm, because the battery space is an exciting one. However, the time scale for developments like these are likely longer than most people realize. Thanks for sharing!
Thanks for your input. Yes I agree - I think this technology is still a long way off being commercial-ready. The research referenced above seems to be aimed at the solution to your first 2 points. And they are doing this using a coating on the anode which they claim allows the lithium ions to pass through while blocking the compounds resulting from the reactions of non-oxygen elements in the air. But they are still at a ~500 cycle limit which needs serious improvement.
Out of curiosity, what is your background? I see from your bio that you are interested in a lot of the same things I am.
Education-wise it was electrical engineering & work-wise it's been telecommunications network design and renewable energy mostly. What about yourself?
Oh nice @rynergy! Education in ChemE and working in electrochem/materials science. I'll have to check out some of your other blogs. If you are interested I just started writing about batteries and I'd love your input!
OK followed. I will have a look. Cheers