[Club 100]| Structure of Cellulose. By @fonjougiresse
Greetings steemians. After the general introduction and history on cellulose here I come with a detailed structure of it.

Designed on Canva
Cellulose Structure
The first work of Braconnot concerning the acid hydrolysis of the constitutive substance of the plant cells goes back to the beginning of the 19th century; it is to Anselme Payen that the merit is to have established that the fibrous part of all plant cells had a unique chemical composition. It is also in the wake of the work of Payen that the word cellulose was born and appears for the first time in a report by the Academy of Sciences. All of this work has led to the establishment of the "Primary" structure of cellulose, which is therefore a linear homopolymer of glucose residues of configuration D, which are connected together.
Cellulose is the most abundant constituent of wood cell walls. This glucose polymer is the main structural element of many plants. Depending on the plant species, the content varies from 40 to 45% for softwoods (soft) to 40 to 50% in hardwoods (hardwoods).
Cellulose contains several structural levels. The different levels are as follows (figure 1):
- The molecular structure of a cellulose polymer,
- The polymers are ordered into microfibrils with crystalline and non-crystalline regions.
- Several microfibrils are assembled together to form a macrofibril.
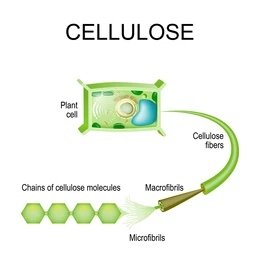
Image Credit figure 1; Cellulose plant cell fibres
The molecular structure
Cellulose is a linear homopolysaccharide consisting of D-glucopyranose units linked by β(1-4) glycosidic bonds. The repetitive unit, composed of the association of two glucoses, is called cellobiose. The latter consists of two glucose units linked by the glycoside bond C1-O-C4.
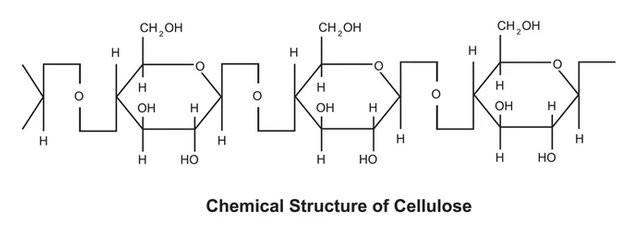
Image Credit
It is noted that the reducing end of the polymer corresponds to the glucose unit whose hydroxide is free. The non-reducing end is so named because the hydroxyl group is engaged in a glycosidic bond.
Cellulose microfiber
Cellulose microfiber is made up of two regions, one crystalline, made of an organised polymer of molecular cellulose chains, and the other, said to be non crystalline, also called an amorphous region, made of unorganised molecular cellulose chains.
At this level of structural organization of cellulose, other than the glycosidic bonds that exist in a chain, we find other hydrogen bonds such as:
An intramolecular hydrogen bond is formed between C2 and C6, with C5 and C3 of the same chain.
*Interchain hydrogen bonds formed between C3 and C5 of a different chain
We should note that, the crystalline structure has many forms that are known: The Iα and Iβ forms
| Organism | Major type |
|---|---|
| Algae and Bacteria | Iα |
| Plants and Tunicates | Iβ |
Cellulose Macrofibrils
A macrofibril is formed by the assembly of cellulose microfibrils linked with each other by hydrogen bonds of the intrachain type. (Same as that found in the cellulose microfibril). A macrofibril is an organised repetition of cellulose microfibrils where all the bonds present are identical to those which are found in a macrofibril.
Next article will be on how the structure is related to it function.
Upvoted! Thank you for supporting witness @jswit.

Please check my new project, STEEM.NFT. Thank you!
Thank you @pelon53. I'll try to be among the best