How does the Earth's mantle melt?
The Earth’s Upper mantle consists of ultramafic rocks. Their main components are olivine and orthopyroxene, as well as clinopyroxene and an Al-phase (plagioclase, spinel, or garnet). Additionally mantle rocks can contain phlogopite, amphibole or carbonate. From these different compositions different mantle melts can be produced, depending on the temperature and pressure conditions.
But how can you even melt a mantle rock?
First we need to understand the position of the solidus (everything is solid) and the liquidus (everything is liquid) in peridotites during mantle conditions as well as the realistic path of the geothermal gradient that could cross the solidus.
The difference between the liquidus and the solidus in dry (so water free) peridotites is roughly 800 °C. At higher pressures of around 50 kbar the difference is only 200-300 °C. In a stable tectonic environment without thermal anomalies the mantle geotherm does not cross the solidus of a dry peridotite, but lies roughly 200 °C deeper (Figure 1). This means, that under normal conditions, no melt is created.
There are generally three possible ways to melt the mantle.
- Increase in temperature.
This can happen if new material is added to the upper mantle from the depth, for example via a mantle plume. - Decompression melting.
This occurs when a rock rises adiabatically (without heat loss) to lower pressures. This is common in fast rising plumes. - Addition of fluids.
The presence of water lowers the melting point of rocks significantly. The water saturated peridotite solidus during pressures above 1 kbar lies 500 °C lower that the dry peridotite solidus and cuts the natural geotherm.
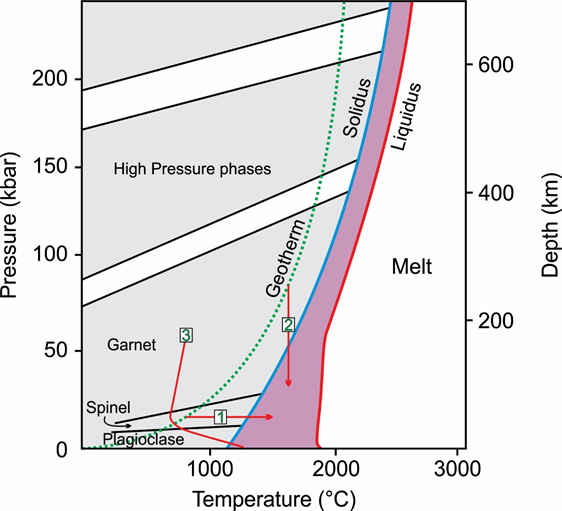
Figure 1: Phase diagram of aluminous lherzolite with geothermal gradient. Numbered arrows correspond to the numbered list in the text. (1) Increase in temperature (2) Decompression melting (3) water saturated peridotite solidus.
After Wyllie (1981).
The third option leaves the question: How can water get into the mantle?
The first option is that water gets released from subducting oceanic crust into the mantle wedge above, which is common in subduction zones at certain depths (Figure 2). With increasing temperature, hydrous minerals in the oceanic slab get dismantled and release fluids. The water can also be stored within the peridotite in hydrous minerals like mica and amphibole, which release fluids during pressure or temperature changes.
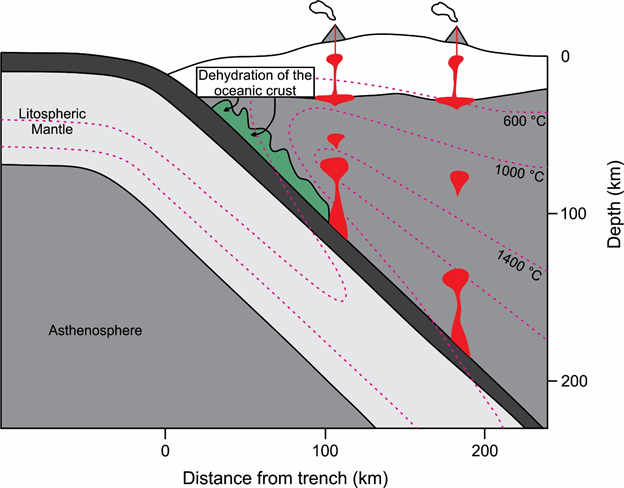
Figure 2: Cross section of a subduction zone showing isotherms and potential magma source regions.
After Tatsumi and Eggins (1995).
Lherzolite never melts completely, but only partly. This partial melting does not mean that all minerals melt by the same amount. Some minerals melt first, and some melt later.
The general melt reaction in garnet lherzolites is:
The stoichiometry of this reaction is highly dependent on the pressure and temperature conditions, as well as the exact composition of the rock.
The degree of melting (how much percent of the solid rock gets molten) dictates the composition of the melt. There are less clinopyroxene and Al-phases in lherzolites than orthopyroxene and especially olivine. Small degrees of melting therefore contain higher amounts of melts from these rarer minerals, whereas at high degrees of melting the composition of melt is dominated by olivine. Small degrees of melting therefore produce melts that are enriched in incompatible elements.
Basalt is a volcanic rock, which contains plagioclase and clinopyroxene. The remaining mineralogical components, as well as the chemical composition, can vary without any macroscopically noticeable changes. These changes can only be defined analytically or microscopically and are important to understand the processes occurring in the Earth’s interior.
Generally we can say:
Melting of a lherzolite forms a basalt and a residuum that is depleted in incompatible elements, for example a harzburgite.
We know that Ca and Al are the elements that first leave the rock to wander into the melt, because basalt is mainly composed of clinopyroxene and plagioclase, so Ca- and Al-rich phases.
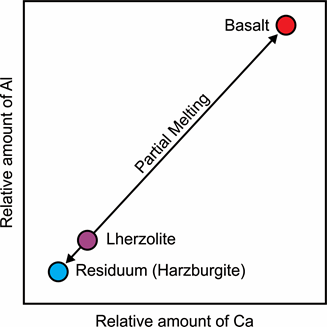
Figure 3: Representation of the behaviour of Calcium and Aluminium during the melting of a lherzolite.
To understand the formation of chemically different basalts, we have to discuss the melting of these two main components of the mantle: Depleted peridotites that have previously undergone partial melting and enriched or fertile peridotites that have not yet been partially molten.
Let’s first look at the depleted mantle. From this type of mantle only different types of tholeiitic basalt can be created. This is relatively independent from pressure and temperature conditions. The degrees of melting always lie between 20 and 30%. It is noticeable that low pressure melts produce quartz tholeiites and high pressure melts produce olivine tholeiites.
Partial melting of enriched peridotites produce tholeiites at 20-30 % degrees of partial melting. Depending on the pressure these are either quartz tholeiites or olivine tholeiites.
Low degrees of partial melting of around 10% on the other hand produce alkaline basalts. These also tend to get more olivine rich at higher pressure. Alkaline basalts have higher contents of incompatible elements compared to tholeiites, because the melt gets separated before the complete amount of clinopyroxene (the main mineral containing the imcompatible elements) is exhausted at higher degrees of partial melting.
The rule of thumb goes: The higher the pressure and the lower the degree of melting the more alkaline the melt.
It is important for the understanding of these processes to note that the absolute amount of incompatible elements in alkaline basalts and tholeiites can be similar, but they are much more diluted in in tholeiites, because the amount of molten material is larger.
At last melt is produced in the Earth's mantle during subduction zone magmatism. We have already discussed the melt production in the mantle wedge due to fluid input by dehydration of the subducting slab. The only thing missing is the melting of the subdued oceanic crust itself. The oceanic crust does not consist of fresh basalt anymore, but additionally contains hydrous minerals (zeolite, chlorite, amphibole) due to reaction with sea water. These hydrous minerals are only stable at limited temperatures and pressure and will continuously release water to form anhydrous minerals. This can lead to dehydration melting. Under such conditions andesites are formed, which are characteristic for island arcs. Different dehydration reactions in the subducting slab lead to different forms of volcanism along the slab (Figure 2).
Sources
- Markl, G. (2008). Minerale und Gesteine, 2. Auflage. Spektrum Akademischer Verlag
- Tatsumi, Y. & Eggins, S. (1995). Subduction Zone Magmatism. Oxford: Blackwell Scientific
- Wilson, M. (1991). Igneous Petrogenesis: A Global Tectonic Approach. Harper Collins Academic
- Winter, J. D. (2001). An Introduction to Igneous and Metamorphic Petrology. Upper Saddle River, NJ: Prentice Hall
- Wyllie, P.J. (1981). Plate tectonics and magma genesis. Geologische Rundschau 70, 128-153
You received a 80.0% upvote since you are a member of geopolis and wrote in the category of "geopolis".
To read more about us and what we do, click here.
https://steemit.com/geopolis/@geopolis/geopolis-the-community-for-global-sciences-update-4
If you do not want us to upvote and comment on your posts concerning earth and earth sciences, please reply stop to this comment and we will no longer bother you with our love ❤️