Importance of the study of thermodynamics in Mechanical Engineering
Public domain Image source. Wikimedia commons
Theoretical bases of the study of thermodynamics.
There are many processes that occur in the industry that need the study and contribution of mechanical engineering, and this within its forms of application, is nourished by thermodynamics to solve many phenomena that involve changes in energy transfers due to changes within the temperatures in different systems involved. For many years the study of thermodynamics has been more attached to science than to engineering, that is from the 1820s onward thermodynamics based its studies on atomic and molecular theories (internal structure of matter) walking through various theories until we reach more practical studies and contributions such as, for example, the possibility of giving a more modern mode and way of life to humanity. Have the following questions ever been asked:
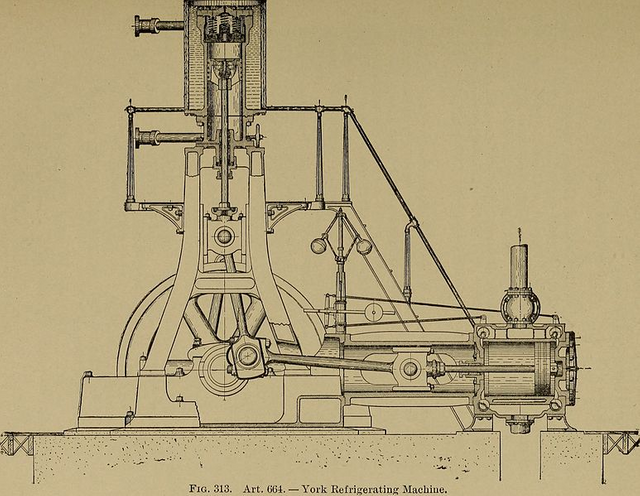
How does a refrigerator cool its contents?
Public domain image source: wikimedia commons
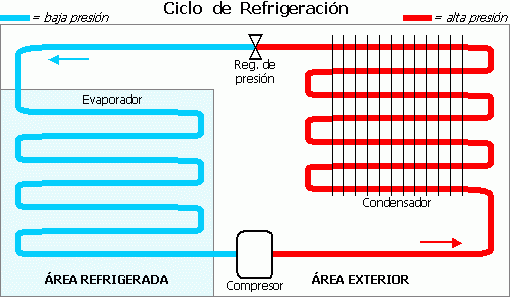
What types of transformations are those that occur in a power plant?

Source of public domain image: wikimedia commons
What happens to the kinetic energy of a moving object when it comes to rest?
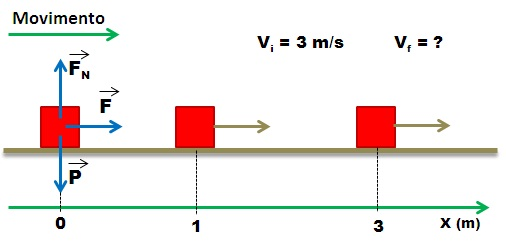
Public domain image source: wikimedia commons
The transformations and evolution that the study of thermodynamics was giving rise to a series of laws that give answers to these questions, and in the process that thermodynamics, apart from being governed by scientific studies, can contribute to engineering.
- Law zero (thermal equilibrium): If two objects A and B are separately in thermal equilibrium with an object C, then A and B are in thermal equilibrium with each other. This law is summarized with the following graphic:
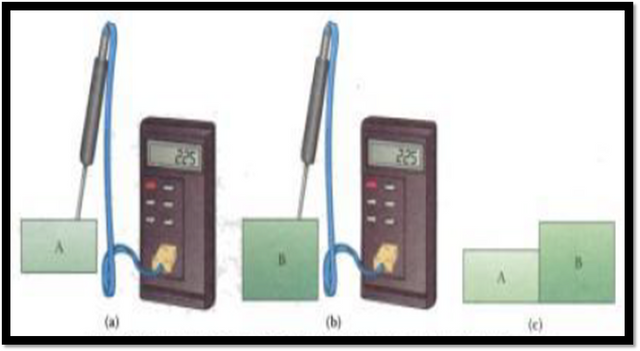
[Image source: physics book. Author: serway. Image edited with microsoft power point tools]
Another summarized and understandable way to understand the zero law of thermodynamics is taking into account that if two objects: A and B are in equilibrium it is because they have the same temperature, otherwise they would have different temperatures if they are not in thermal equilibrium.
- First law of thermodynamics: This is a law that can be applied to numerous processes within mechanical engineering, and at the same time provides a link between the microscopic and macroscopic.
The first law of thermodynamics is a special case of the law of conservation of energy, which includes two stages:
- Changes in the internal energy of matter.
- Transformation of energy by heat and work.
To understand a bit how the first law works, I will explain the following example:
- Suppose we have a gas at initial conditions whose pressure and volume would be initial pressure and initial volume, this gas is subjected to a temperature gradient in which it acquires a final state of final pressure and final volume.
It is obvious that during this process the gas underwent a change, where there was a transfer of energy to the system by means of heat, a process by which work was also carried out on the system.
It can be concluded that the work plus the heat transfer in the initial and final state change is completely determined by the initial and final states of the system and will be equal to the internal energy manifested by the system.
Which means that the whole first law can be summarized in the following equation:
Where:
ΔE = Internal energy of the system
Q = Heat transfer
W = Work done by the system
Application of thermodynamics in mechanical engineering. (personal perspective).
There is no process that is executed within the industries, where there are no transfers and energy transformations, as mentioned at the beginning of the article with the three questions of analysis that were made, the transformation of electrical energy needs a science such as thermodynamics to be able to understand all those thermal and heat transfer processes. The thermoelectric converts the heat generated by diesel fuel into electricity, in all this process is of vital importance the fundamental principles of the laws of thermodynamics, especially to understand what there must be a balance in energy transfers .

Public domain image source: wikimedia commons
Conclusion
Transcend in the study of thermodynamics to bring industrial processes in the best way, more optimized, especially in the industrial field where there are boilers, turbines, evaporators, condensers, cooling towers, large-scale combustion processes. Knowing how to handle and apply the laws of thermodynamics we will improve these processes, which in turn infer in the improvement of production systems.
The mechanical engineer as a designer, supervisor and evaluator of various projects that involve being a maintainer, must have clear knowledge and application in thermodynamics, especially taking into account that already in the workplace is much lost academic sense, and many processes are they let vitar by the habit to solve the problems, and the academic thing is left of the hand, and in this particular case the thermodynamics like fundamental axis within the Engineering in general and still more in the mechanical engineering.
References:
Physics for science and engineering. Volume I. Raymond A. Serway. Jhon W. Jewett Jr. Sixth edition.
Congratulations @ajfernandez! You have completed the following achievement on the Steem blockchain and have been rewarded with new badge(s) :
Click on the badge to view your Board of Honor.
If you no longer want to receive notifications, reply to this comment with the word
STOPDo not miss the last post from @steemitboard: