Three-Process Thermodynamic Cycles | Review Problem (SI Units) | Part 2 of 2
Hi folks!
Previously I had made an article which demonstrates on how to analyze and solve thermodynamic cycles which is composed of 3 distinct thermodynamic processes and I also included with it a review problem which is expressed in English units which is at the same time I choose to make it as Part 1 of 2.
So this time around, I'll be showing another three-process thermodynamic cycle wherein it is now expressed in SI units. And without further ado, here is the review problem:
A three-process cycle of an ideal gas, for which cp =
1.064and cv =0.804 kilojoules per kilogram per degree Kelvin, is initiated by an isentropic compression 1-2 from103.4 kiloPascal,27 degree Celsiusto608.1 kiloPascal. A constant volume process 2-3 and a polytropic process 3-1 withn = 1.2completes the cycle. Circulation is a steady rate of0.905 kilogram per second, compute for:
Heat Added, QANet Work done by the cycle, WNCycle efficiency, eMean Effective Pressure, PME
In obtaining all those important parameters that are being asked by the review problem, the very first thing to do is to draw the Pressure versus Volume Diagram (P-V Diagram), which is found below.
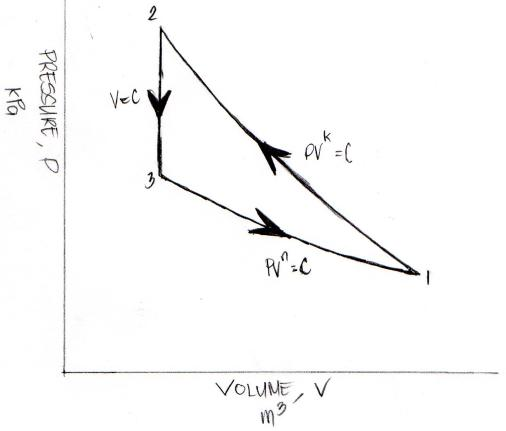
After the plotting the P-V diagram of the said review problem, to make it appear as pretty straightforward, I am going to compute all the needed parameters in computing the 4 parameters being asked by the review problem. So what you will be able to see in the later part of this review problem demonstration is more of substitution of the needed parameters like temperature (T), volume (V), adiabatic index (k) and the polytropic specific heat capacity of the ideal gas (cn).
So I will begin obtaining the adiabatic index of the ideal gas (k) which it experienced during the isentropic compression of process 1-2. And the computation goes like this:
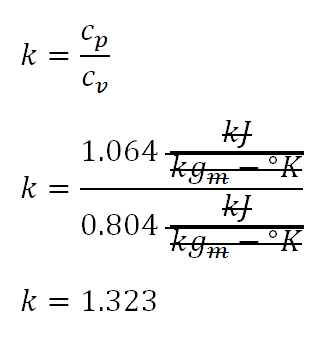
Based on the above computation, the adiabatic index of the ideal gas at process 1-2 is equal to 1.323.
Next parameter that I choose to obtain is the temperature at the end of isentropic compression (T2) of process 1-2. And the calculation is found below.
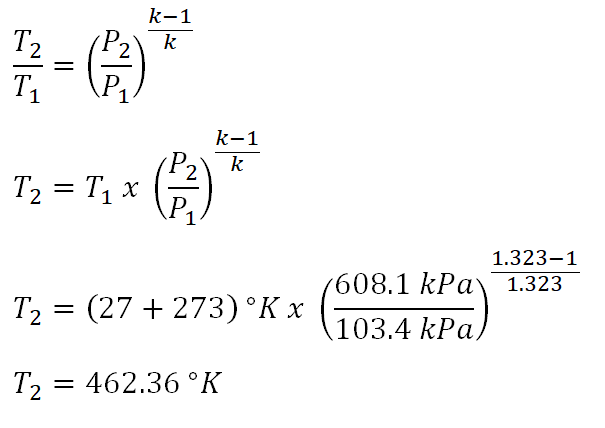
The temperature at the end of isentropic compression is equal to 462.36 degree Kelvin.
Third parameter to obtain is the gas constant of the ideal gas (R), which can be obtained since we are provided with the data for cp and the cv. And the calculation looks like this:
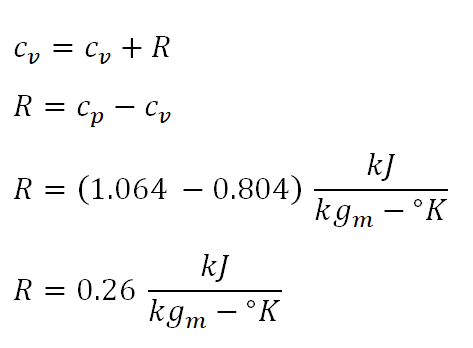
The gas constant of the ideal gas is equal to 0.20 kiloJoules per kilogram per degree Kelvin.
Fourth parameter to obtain is the volume at beginning of isentropic compression (V1) which can be obtained using the formula for the universal gas constant. And the computation goes like this:
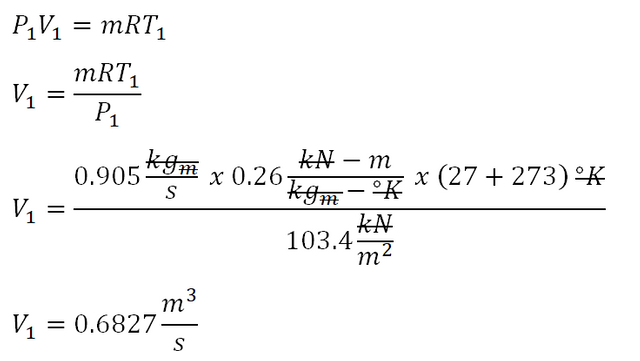
The volume of the ideal gas at the beginning of the isentropic compression is equal to 0.6827 cubic meters per second.
Fifth parameter to obtain is the volume at the end of isentropic compression (V2) which can be obtained using the relations of pressure (P) and volume (V) in an isentropic process. And the computation goes like this:
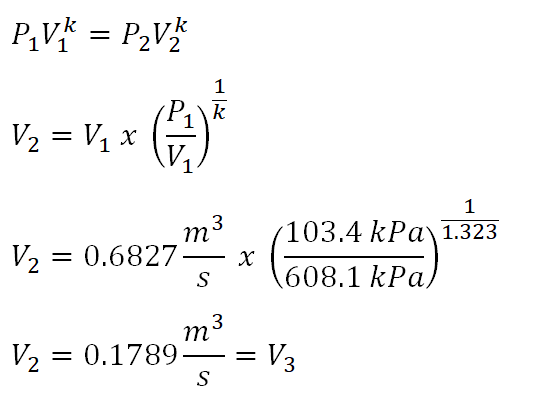
The volume at the end of isentropic process is equal to 0.1789 cubic meters per second. And since process 2-3 is a constant volume process, thus V2 is just equal to V3.
And the sixth parameter to obtain is the temperature at the beginning of process of process 2-3 (T3) and the computation goes like this wherein it uses the polytropic relation of temperature (T) and volume (V).
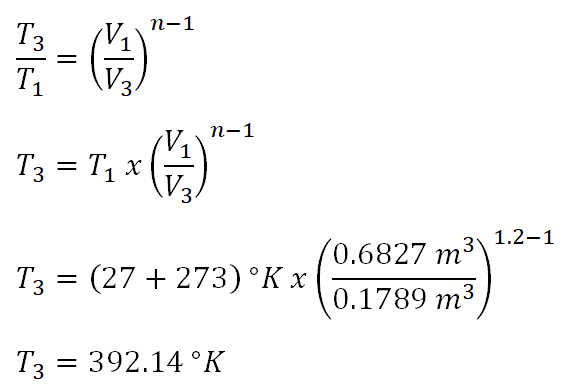
The temperature of the ideal gas at the beginning of polytropic process is equal to 392.14 degree Kelvin.
And the last important parameter to obtain that is useful in obtaining the parameters that are being asked in the review problem is the polytropic specific heat capacity of the ideal gas (cn) and the computation goes like this:
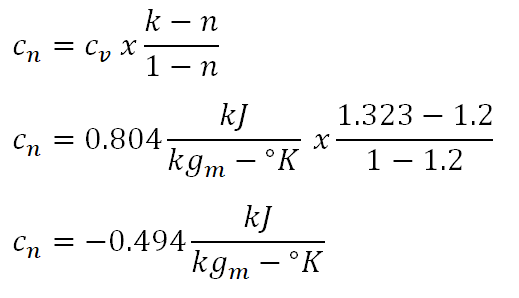
The polytropic specific heat capacity of the ideal gas in this cycle is equal to -0.494 kiloJoules per kilogram per degree Kelvin.
As mentioned a while ago that the computation for the parameters that are being asked in the review will now be more on substitution and now let us now start the computation.
Heat Added, QA
We couldn’t identify on what process is the heat being added so with that the only way to obtain the heat that is being added to cycle is to compute the heat per process.
So starting with Process 1-2, for isentropic process, there exists no transfer of heat. Thus heat added is equal to zero.
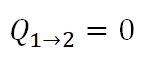
During isentropic process, Q is always zero.
Next is Process 2-3, for isometric process, the computation of heat goes like this:
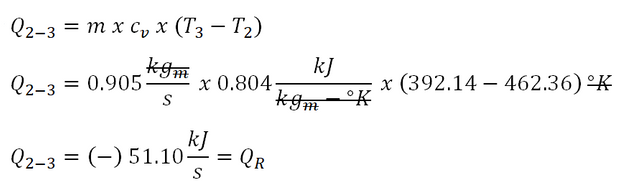
Upon computation we have obtain a heat that is equal to -51.10 kiloJoules per second and that negative sign indicates that the heat is being rejected and is being done by the ideal gas.
Finally, Process 3-1, for polytropic process, the computation for the heat goes like this:
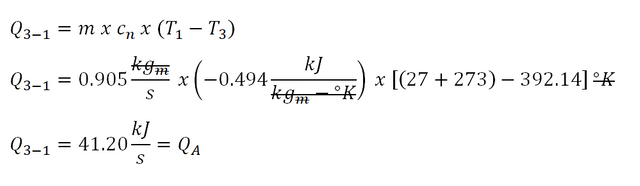
Based on the above computation, the heat during the polytropic process is equal to 41.20 kiloJoules per second and that means that it is the heat being added to the cycle.
Thus the heat being transferred to the cycle is equal to 41.20 kiloJoules per second.
Net Work done by the cycle, WN
Since we have obtained the values of the heat being added and being rejected during the cycle, we can directly obtain the net work done by the cycle. And the computation goes like this:
The net work of the cycle is equal to
-9.9 kiloJoules per second. Take note of thenegative signsince that indicates that the work is beingbythe ideal gas to the surrounding.
Cycle efficiency, e
Since we have obtain the necessary parameters for the computation of the cycle efficiency (c), we will substitute it directly to formula and the computation goes like this:
The efficiency of the cycle is equal to 0.2403or24.03%.
- Mean Effective Pressure, PME
For the mean effective pressure, we can just directly substitute the values of the volumes (V1 and V2) and the WN to the general formula. And the computation goes like this:
And with calculation being shown above, we have obtained the PME of the cycle that is equal to
19.65 kiloPascals (kPa).
So we have obtained all the parameters being required from the review problem. It's a quite easy and I hope you learnt something from this presentation that I have prepared.
Thank you for spending your precious time reading this blog.
Much love and respect.
Ace | @josephace135
Reference:
Hipolito B. Sta. Maria, Thermodynamics
Solutions are being made by me and the sample review problem is found in page 92, problem number 5.
The above PV diagram for this review problem was manually made by me in preparation for this article.
Presentation of equations and formulas were made possible by MS Word 2010 Equations function.
Screenshots were made possible by utilization of Snipping Tool application.
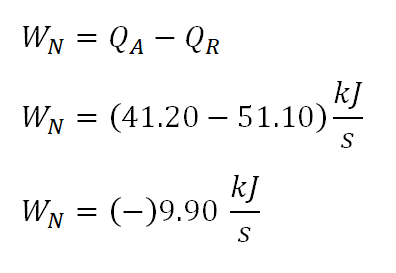
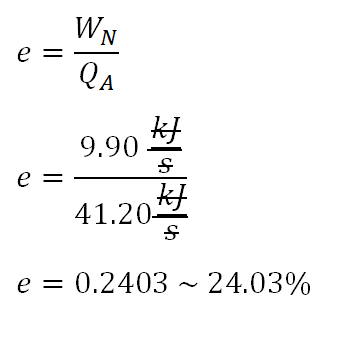
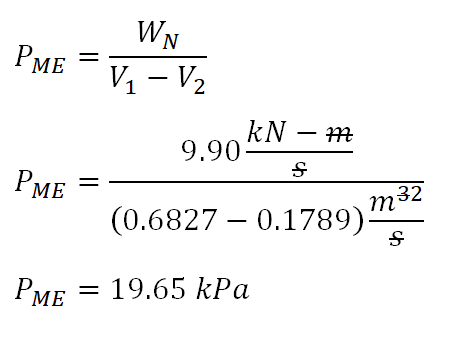
Just curious, how u add the formula onto the post?
Hey bro @superoo7, I didn't add the formulas in this post, those are screenshots being made using Snipping Tool. Been searching for quite sometime on how to make mathematical equations in Steemit markdown.
maybe can try LaTeX, I think LaTeX can convert to image, haha
Check this http://latex2png.com/
Wow! This is really great. I'm going to have that link bookmarked. Imma use it in my future contributions for STEM related problems. Thank you so much bro @superoo7!
I am still trying to learn LaTeX haha, maybe will create tutorials.
yay! I might follow the same thing as yours ;)
You have been upvoted by the @sndbox-alpha! Our curation team is currently formed by @bitrocker2020, @zord189, @aaronleang, & @teachblogger . We are seeking posts of the highest quality and we deem your endeavour as one of them. If you want to get to know more, feel free to check our blog.
You received an upvote as your post was selected by the Community Support Coalition, courtesy of @steemph.antipolo
@arabsteem @sevenfingers @steemph.antipolo