Chemistry?
EVER WONDER WHY THE PERIODIC TABLE IS SHAPED THE WAY IT IS?
A quick lesson: The shape of the periodic table can be explained perfectly through quantum mechanics. This sounds complicated but if we ignore the wildly confusing mathematics involved we can understand it with three basic principles. First, pairs of electrons go into orbitals, and each orbital has an energy. Second, the lower energy orbitals fill up first. Third, electrons are needed cancel the positive charge of protons. With this we can clearly understand why the periodic table has the shape that it does.
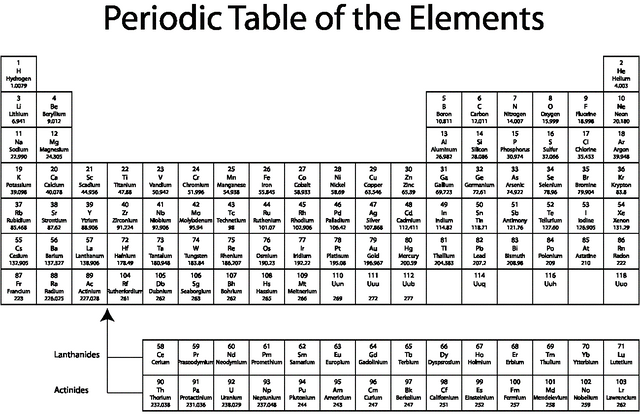
Orbitals come in blocks based off the principle Quantum number. If you look at the periodic table below, the very top row we have hydrogen, a space and helium this is where the principle quantum number is equal to 1. We know that hydrogen has one central proton with a positive charge, so it naturally wants to attract one electron to cancel out the charge. Similarly helium has two protons and wants two electrons.
For a principle quantum number 1., we have an orbital number equal to n-1, where "n" is the principle quantum number. This orbital is "0", and is called the "S" orbital.
Each known orbital can hold two electrons. As the nucleus increases in the number of protons, more orbitals are needed to hold the electrons that are used in canceling out the positive charge.
Now lets move beyond helium. If we go to three protons, and three electrons, we need another set of orbitals. We now are looking at lithium. This has a principle quantum number of 2. What this means it that we now have two sets of orbitals. where we can use the equation "n-1" twice thus we have, "1", and "0" orbitals. We have already established that "0" is an "S" orbital. But we are now in the principle quantum number two, so we will call it the "2S". Since we established that lower energy orbitals fill up first we can conclude that the "2S" is a higher energy orbital. The "1" orbital requires some more math to understand, but its simplified.
"1" is the "P" orbital, since this first occurs at the principle quantum number two, we call it the 2P orbital. As orbitals increase in number they increase in complexity. The "2P" orbital has several locations to hold electrons. The locations are based on the orbital number, and can be calculated by subtracting one, until "-1" is reached with each whole number representing an orbital, and "0" is included. What this means for "P" orbitals is we can have[ -1, 0, +1] individual orbitals in the "P" block.
Each whole number is an orbital, and we can always have a "0" orbital.
So, for principle quantum number 1., where the orbital number is "0" we have only one orbital and that is called the "s" orbital. See chart below.
For principle quantum number 2., we have two blocks of orbitals, the "S" and the "P" we know that the "S" is "0" orbital, and P is "1" orbital. Thus a "0" orbital has only [0] orbitals present, however, the "1" orbital has a [-1,0,+1] that is a total of three orbitals.
With three orbitals we can hold six electrons, so we can see a group of six atoms, on the right of Lithium. This is the "P" block on the periodic table, and as one has guessed the section to the left is the "S" block.
At this point we have covered some pretty heavy material, so we will use what we know so far and finish describing the periodic table. But first we have to discuss energy.
The more complicated orbitals are higher in energy, so it is not uncommon for a 4S orbital to be lower in energy than a 3D orbital, or a 4D be lower in energy than a 4F.
The next part of the periodic table starts at atom 21, scandium. Here we count to ten ending at zinc. Thats ten protons, which means ten electrons need a space, which refers to a total number of five orbitals. This is called the "D" block
The lower section has a count of fourteen, which is equal to seven orbitals. This is called the "F" block.
Hear is a simple scheme to understand the numbers.
- "0" [0] 1S
- "1", "0" [-1,0,1], [0] 2P, 2S
- "2", "1", "0" [-2,-1,0,1,2], [-1,0,1], [0] 3D, 3P, 3S
- "3", "2", "1", "0" [-3,-2,-1,0,1,2,3] [-2,-1,0,1,2], [-1,0,1], [0] 4F, 4D, 4P, 4S
So now we see that each number in the brackets represents its own orbital where a pair of electrons can be placed. As more electrons are needed larger orbital systems come into play.
So one final note on energy to clear up why we don't see the 3D orbitals until after the 4S orbitals have been filled. First lets look at the atoms we are discussing, so to find the 4S, we look for the 4th row, thats potassium (K). Logically we would expect the 3D orbitals to fill up first, however the 4S is lower on the energy scale due to its simplicity, having only an "S" orbital, so it will fill up its two electrons first, then the 3D will get its electrons. Thats why we see the D block in the middle of the table.
The F Block is even higher in energy, and not observed until after the 6S. So in the energy scale 6S fills first, then the 4F fills, then the 5D fills. To test if you understand try to label all the electron orbitals present in atom number 79, Gold!
In conclusion, the periodic table layout follows some fundamental principles, and quantum mechanics. The lowest energy orbitals get filled by electrons that are attracted to the positive charge of the protons on any one atom. As more electrons are needed more orbitals are needed, and the entire chart is based on what energy level each orbital is.
Some food for thought, why do iodines, like to be iodide, and sodium wants a plus charge? Mull it over, and respond back!
Cheers, Gilley
P.S.
This is my first Chemistry post, I am sure the next ones will be better, so stay tuned. I am willing to take request if you have a specific chemistry question let me know!
Hello @gilley,
Congratulations! Your post has been chosen by the communities of SteemTrail as one of our top picks today.
Also, as a selection for being a top pick today, you have been awarded a TRAIL token for your participation on our innovative platform...STEEM.
Please visit SteemTrail to get instructions on how to claim your TRAIL token today.
If you wish to not receive comments from SteemTrail, please reply with "Stop" to opt out.
Happy TRAIL!
Yep! There is stability in having completely full octets. Its called an octet because there are 8 total electrons involved. Follow up question why does Iron like to have a 2+, or a 3+ charge? Think about the orbitals and the electrons to figure this one out.
The electron configuration of iron is: [Ar] 4s2 3d6.
After that we have a stable configuration: [Ar] 4s0 3d5. Argon is stable (the octet); d5 is stable as well (half full d-orbital).
Yep, obviously, a chemist at heart, if not in profession.
You could have added the #science tag I think ...
Congratulations @gilley! You received a personal award!
You can view your badges on your Steem Board and compare to others on the Steem Ranking
Do not miss the last post from @steemitboard:
Vote for @Steemitboard as a witness to get one more award and increased upvotes!
Congratulations @gilley! You received a personal award!
You can view your badges on your Steem Board and compare to others on the Steem Ranking
Do not miss the last post from @steemitboard:
Vote for @Steemitboard as a witness to get one more award and increased upvotes!