Chemistry Lesson: Part 11 (Atomic Structure Part 2)
Original Image Source
Introduction
This series of posts seeks to present the material covered in the first semester of a college level general chemistry course, in an easily digestible steemit blog post format. The series is intended to be read, and experienced in sequential order starting with Post 1. The material will build upon itself, and potential exercises included (problem sets), will pertain to the post they are contained in, or any previous post. Each post will pick up immediately where the previous in the series left off. Please check out the #chemistry-lesson tag for all posts in this series. I hope you find this series to be informative and beneficial toward your understanding of chemistry and science in general.
Immediate Preceding Post
Part 10: Atomic Structure Part 1
Re-Introduction from Part 10
In the previous post we discussed a buildup to Niels Bohr’s model for the hydrogen atom. We talked about light, and how increasing understanding of the ways light exists, in turn, lead to a basic understanding of electrons and the atom. Of course other experiments were done previously to understand the nucleus and that electrons existed, but Bohr’s experiments and determinations gave us the clearest picture (to that point in time) of what an atom truly looked like. Today we will continue to dive into the structure of atoms, and discuss a bit of Quantum Mechanics. I will discuss this from the perspective of a General Chemistry course, and will likely continue to make simplifications over the level of detail covered in a more physics based course. Nevertheless, I hope you will find the complexity level that we will go into enough to challenge your mind, but not too much so as to leave you in the dark.
Quantum Mechanics
Bohr’s Methods Don’t Work For Atoms Other Then Hydrogen!
If you recall, the model that Neils Bohr came up with for the hydrogen atom looked like this:

Unfortunately, the method that Bohr used, could not explain the emission spectrum for any other elements (aka if it had more than one electron as all elements other than hydrogen do, it no longer made any sense!). At the time scientists were perplexed by all of this. They were additionally perplexed by work done by a few scientists that showed that not only were electrons particles, but they also (like light) behaved like waves. The experiments those scientists did were to shoot a beam of X-rays at some aluminum foil and watch the pattern produced on a screen placed behind the foil. The X-rays made a concentric circular pattern on the screen which is indicative of wave like behavior (no surprise because X-rays are waves). The scientists then shot a beam of electrons at the foil, and to their surprise they saw a similar concentric circular pattern… inciting that the electrons were also able to behave like a wave.
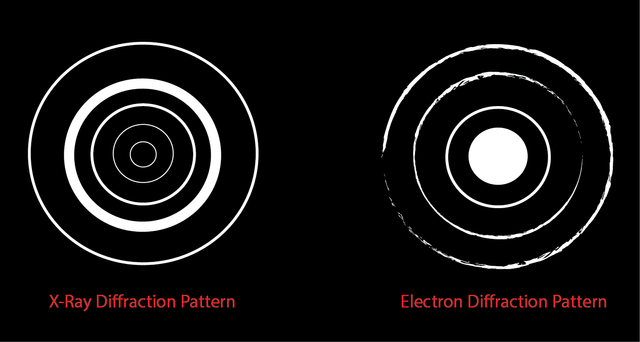
This discovery (that electrons were wave-like) also confused scientists, as the precise location of a wave cannot be defined (waves extend outward into space, they are not tightly confined to a specific location). A German physicist came up with a description for this problem, his name was Werner Heisenberg:


The description for the problem he came up with is called the Heisenberg uncertainty principle and it states that the more precisely we know the position of an electron, the less precisely we know its momentum (momentum is velocity multiplied by mass). (Cite) Based on this theory scientists concluded that it is not appropriate to imagine an electron orbiting a nucleus in a defined circular path (as Bohr proposed). Another scientist, Erwin Schrödinger came up with a mathematical representation to better describe the behavior of these subatomic particles (that equation is called the ** Schrödinger Equation**, and we are not going to discuss it directly, its honestly a topic that one only dives into in advanced chemistry/physics courses! We will discuss what it tells us about atoms though.)
What Does The Schrödinger Equation Tell Us?
The Schrödinger equation tells us about all of the possible energy states that an electron can reside in, and these are described by something called Quantum Numbers. Additionally this equation presents to us the concept of “electron density” as rather than orbiting in a specific circle the electron has a certain probabability of being located in a spot around the nucleus at any time. Some spots have a higher probability of the electron being there then others (those spots are closer to the nucleus). The electron density model changes how we picture an atom, from Bohr’s depiction above with the orbits to this:
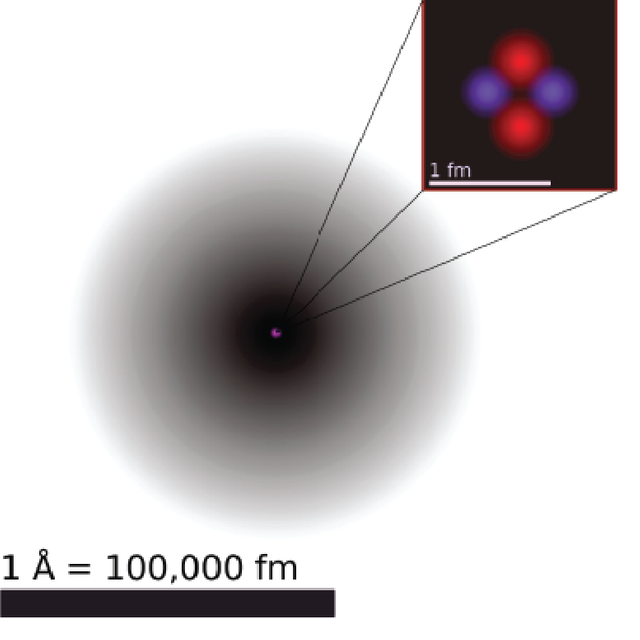
Where the nucleus is in the center, and the dark shaded region surrounding it is the electron density, the darker the shading, the more likely an electron is to be in that region at any particular instance in time. Remember because of the Heisenberg uncertainty principle we do not know exactly where the electron is, so it is represented by this probability distribution. Now rather than discussing an electrons orbit around the nucleus we talk about “atomic orbitals” which are shapes defined by the electron waves and confine where the electron resides.
Quantum Numbers
I will end today’s blog with a discussion of quantum numbers, and pick up the next blog talking about how these quantum numbers relate to atomic orbitals and electron configuration. So what is a quantum number? They are the method that we use to describe the distribution of electrons in an atom. These values are determined from the solution to the Schrödinger equation for the Hydrogen atom (and approximations are used to apply these values to atoms with more than one electron). There are four quantum numbers (angular momentum, principal, magnetic, and spin). These numbers describe the atomic orbitals and the electrons that are inside of them. Let’s talk about each one:
Principal Quantum Number
The principal quantum number or n, has whole number integer values (1, 2, 3…etc), if you recall back to the previous post where we discussed the energy of a hydrogen atom, we had an equation to define it:

In that equation, n was the principal quantum number. This number defines the total energy of an atomic orbital. This number also shows us the average distance of the electron from the nucleus, where the larger n is the further the electron is from the nucleus.
Angular Momentum Quantum Number
The angular momentum quantum number or l tells us the shape of an orbital. The value of l is equal to any number from 0 to n-1, so lets say n = 2, then l can be equal to 0, or 1. When we are talking about the values of l we generally don’t describe them with numbers, but rather letters:
| l | Orbital Name |
|---|---|
| 0 | s |
| 1 | p |
| 2 | d |
| 3 | f |
When we discuss orbitals, we will talk about things like s orbitals (those are orbitals where l = 0) or p orbitals (orbitals where l = 1). When we talk about n (the principal quantum number) we are talking about all of the various energy states an electron can occupy, this is the “energy shell.” However there are levels within that over all energy shell that electrons can sit at, these “sub shells” are what we group into the orbitals. This may not make sense right now, but be patient with me. It will (hopefully) become more clear after the next blog (I know, this stuff is complex, and you may have to reference back and forth between this blog post and the next one to understand this more completely).
The Magnetic Quantum Number
The magnetic quantum number or Ms is what describes the orientation of the orbital (l) in space, the values of Ms range from –l to +l. So if l =2 then Ms can be equal to any of the following numbers (-2, -1, 0, 1, or 2). This is another concept that will make more sense later, once I have drawn for you the various shapes of the orbitals! For now just know that the values for Ms are determined from the angular momentum quantum number.
The Electron Spin Quantum Number
Finally a simple quantum number, electrons are spinning on an axis (like the earth spins on its axis), and they spin in one of two directions: clockwise or counter clockwise. A spinning electric charge produces a magnetic field, which is why electrons can be effected by magnetism! The two possible directions for an electrons spin are defined by the electron spin quantum number Ms which can be either + ½ or - ½ depending on whether the electron is spinning clockwise or counterclockwise respectively.
Concluding Remarks
As I have said, in the next post we will go into more detail about atomic orbitals, and I will include images to help relate these quantum numbers to shapes which you can use to better visualize the concepts. I hope this post at the very least has helped you begin to get a bit more of an understanding the craziness of the work that was done in the past to get a better understanding of what atoms were, and also the crazy complexity that has been found. Generating a picture of the subatomic landscape is a monumentally difficult task, as these particles are far too small for us to effectively see with our own eyes. Thus the techniques used to gain this understanding are all indirect, and much of the interpretations are based upon mathematics.
Also keep in mind that much of what I have described to you isn't quite right, science is an iterative discovery process. New ideas are built upon the old and as we understand things better, the older ideas get replaced. Nevertheless, it's still valuable to go back and build our own personal knowledge starting from the ground on up!
Future Posts
Subsequent posts will cover: Electronic Configuration of Atoms, Chemical Bonding, and Molecular Geometry, and more.
Reference Figure: Periodic Table
Other References
Constants and Conversions List
Source for Additional Constants
Some Common Ions
An Open Source Chemistry Text Book
Additional Sourcing Information
General Chemistry: Principles, Patterns, and Applications
General Chemistry: The Essential Concepts
General Chemistry
http://dev.physicslab.org/document.aspx?doctype=3&filename=atomicnuclear_davissongermer.xml
http://history.aip.org/exhibits/heisenberg/p08.htm
http://chem.libretexts.org/Core/Physical_and_Theoretical_Chemistry/Quantum_Mechanics/10%3A_Multi-electron_Atoms/Quantum_Numbers
If you like my work, please consider giving me a follow: @justtryme90. I am a PhD holding biochemist with a love for science. My future science blog posts will cover a range of topics in the biology/chemistry fields.
Thank you for your support of my work!
Thank you for supporting #steemprentice!
Shared on twitter
I will always support #steemprentice, helping new users learn the platform is among the most important things that the community can do.
Disclaimer: I am just a bot trying to be helpful.
This would be an interesting series!
Thank you! Check out the main tag for the rest of the series thus far!
A very good post on quantum mechanics! I should hire you to write the next ones of my blog :)
I have one more of these to write (talking about atomic orbitals), I look forward to getting back to more standard chemistry topics. I will leave most of QM to you to write about lol :) You definitely know it better than me!
The matter is time. You know that, don't you? :)
Excellent information...but you made it more interesting with "The other Heisenberg", it pulled my laugh!!
When I think about that principal, in my mind I imagine Walter yelling "Jesse, we need to cook!" I also used to include as many example problems using methylamine as I could back when I taught Gen Chem, I don't know if the students got the reference, but I sure as heck loved breaking bad!
Glad you enjoyed the blog!
Me too, one of my favorite one indeed!! I still remember the first and second episode!! If I were writing about chemics I would also use this kind of examples!
Following for next "episodes" :)
Upvoted and resteemed!!