How Cell Membranes Form Spontaneously
Introduction: Keeping it together
Have you ever wondered what makes you an individual? There might be several reasons, but some of it has to do with the fact that you don't just bleed into other organisms and people around you. If you did that, hugs would be awkward: you'd walk away from a good warm hug carrying somebody else's head, and they'd have your arms...it'd be a mess. Something has to keep your insides inside, and your outsides outside.
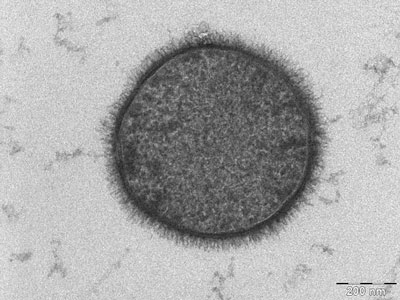
TEM micrograph of a B. subtilis cell. No bleeding out: keeping it all in the family!
Source: Wikimedia Commons contributors
Is it your skin? Well, if you look closer, your skin is just an organ in a system of organs called the integumentary organ system [1: intro]. And the skin is further comprised of tissues — 7 of them, in fact [ibid.] And then there's cells...
Actually, that's where I want to stop. The cell. It's the smallest thing that can be called living, and the smallest living thing that can individuate itself: separate its insides from its outsides. We — macrohumans — are just collections of cells, so naturally we inherit their properties.
So maybe if we can understand how cells keep it — their inner goo — together, maybe we can learn how we keep it together.

Let's find out how we managed to avoid this poor man's fate.
Source: giphy
A chemical detour
I profusely apologize. I really do. But think of it this way: at least it's not math! And, it's a detour, not a main destination. It'll help us better appreciate our final destination (don't worry, no one's getting killed), which is biology. So yay!
6 out of 118
Currently, there's a hundred and eighteen (that's 118) elements in the periodic table. Biologists mainly care about 6 (that's six) of them [2: chapter 2], and only 4 of them — carbon (C), hydrogen (H), nitrogen (N), and oxygen (O) — make up more than 96% of an organism's weight [3]. So already you're getting a sweet deal. Here they are, the magnificent 6:
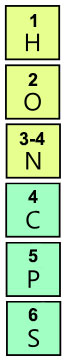
Is it me, or does the N shift a pixel to the right?
Source: Wikimedia Commons contributors, modified.
Shrewd chemists will notice I've replaced the atomic number with the number of covalent bonds the atoms can form. That's because that's one of the numbers biologists most care about [2: chapter 2, section 3].
Now what are covalent bonds, you ask. I won't go into the chemical details, but covalent bonds are basically what hold atoms together strong enough to form larger units called molecules, and those are essential for life [ibid.] Water, after all, is a collection of water molecules, and we are 70% water [2: chapter 2, section 2]. Remember the dude up there in the gif?
Atoms can form many bonds, not just covalent ones. They have a kind of chemical romance thing going on. They can form ionic bonds, and hydrogen bonds [2: chapter 2, section 1], and I won't go into all that. They're just bonds, keeping atoms together. But if you want to remember the number of covalent bonds these 6 atoms can form, here's some useful mnemonic devices.
You can skip this section
(See 3 in references for the number of covalent bonds these atoms use.)
Here's the mnemonic devices I use. Hydrogen (H) and Oxygen (O) are easy to remember: together they form one of the most important molecules for life, H2O. 2 Hydrogens covalently bond with 1 Oxygen, because Oxygen can form 2 covalent bonds, and Hydrogen can only form 1.
Nitrogen is a shifty one. Another word for shifty is coNNiving. Let's just straight out memorize this.
Carbon (C) can form 4 covalent bonds, and if you can remember the explosive C4, you're set.
Phosphorus (P) can form 5 covalent bonds. The number 5 in Greek is pente. That's where pentose (5 carbon sugar) gets its name. Or pentecostal. Or pentachord. There's probably simpler words out there that begin with penta-, but I'm not here to make your life easy. P = pente = 5, is what I'm trying to say.
Sulfur (S) can form six covalent bonds. Now what's a word that begins with the letter s and denotes the number six? Hmm. Let's go with Sistine Chapel. Everyone knows it gets its name from Pope Sixtus IV. It's obvious how the number 6 relates to that name: if you add II to IV, you get VI. So Sulfur can form VI covalent bonds. Best. Mnemonic. Device. Ever. And I'll be damned if you can find a better one.
Greediness
(For all discussions from hereon about polarity and electronegativity, see 3 in references.)
You know how some kids want everything for themselves, whereas other kids are more likely to share their toys? Turns out, that's true of atoms too. Some atoms are more electronegative — excuse me, I meant greedy, I used the proper term there for a second, let's call it an academic slip — greedier than others, so they form polar bonds. Remember H2O? That's a polar covalent bond, because the Oxygen is more electronegative — arg! damn you, books! — because the Oxygen is greedier than the hydrogens.
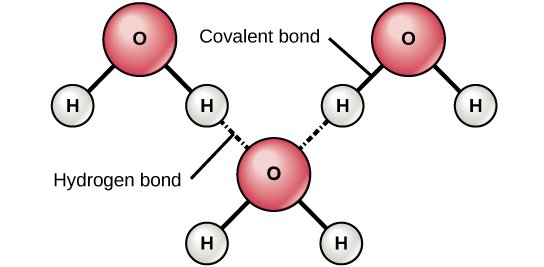
Source: OpenStax, Concepts of Biology
Bonds that share equally are called nonpolar bonds. The bond between a Carbon and another Carbon for instance (represented like so: C—C) is a nonpolar bond. Why? Because logic: they're the same exact atom, so they have the same exact properties, so they can't be greedier than their partner.
But also, they're just not very electronegative even when they're not partnered up with their equals. That's just how the Carbon rolls.
Rule of thumb: when a molecule has Oxygens or Nitrogens, it's polar. When it has any of the rest of the magnificent 6, it's nonpolar.
Polar and nonpolar don't mix
Now what happens when you're a nonpolar bond, and you find yourself in the midst of a bunch of water molecules wanting to interact with one another, the Oxygen orienting itself so it can form hydrogen bonds with Hydrogens from other water molecules, like the picture above?
Well, let's think of it macrohumanly. What would happen if you were in a crowd where everyone wanted to hug each other, but no one wanted to hug you? Exactly: you'd be slowly pushed to the outside of the group.
Hey, did I just explain why water and oil don't mix? I guess I did!

I'm so Greek, if that were olive oil, I'd squeeze some fresh lemon juice in it, and drink it.
Source: Wikimedia Commons contributors
Another name for polar and nonpolar
Polar molecules like to interact with water. They are welcomed, because they can hug. So they're called hydrophilic (hydro meaning water in Greek, philic meaning loving). Salt can dissolve in water, because it's hydrophilic.
Nonpolar molecules don't like to interact with water. So they're called hydrophobic (phobic meaning fearing).
Fence-sitters
Some molecules just don't wanna commit. They're rebels like that. Part of them is philic, part of them is phobic. We call these amphipathic. I'd call them bipolar, but someone got there before me and planted his moon flag.
We now got all the chemistry we need to proceed with the biology! Detour over! Yay!
By the way, in case you're wondering why all this insistence on water, consider this quote from the Molecular Biology of the Cell: "Life on Earth began in the ocean, and the conditions in that primeval environment put a permanent stamp on the chemistry of living things. Life therefore hinges on the properties of water." [3]
Now we know!
Amphipathic
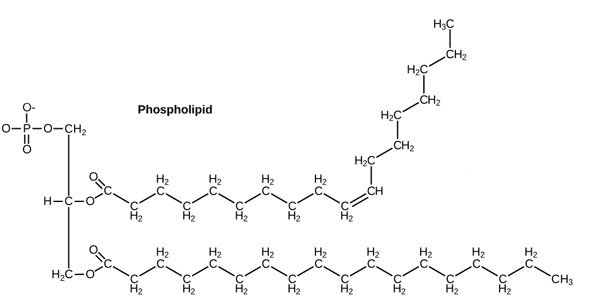
Source: OpenStax, Concepts of Biology, modified.
Now you know enough chemistry to know that the right part of this picture, with all those Carbons and Hydrogens, will be very no-thank-you toward water, whereas the left part of this picture, with all those Oxygens, will be very-happy-indeed-thank-you-very-much to interact with water.
Let's simplify that picture a little bit cos it looks scary. Let's make the philic part a happy smiley face (officially called a phosphate head), and the phobic part some kind of party-pooping black running-away line (officially called a tail) [4].
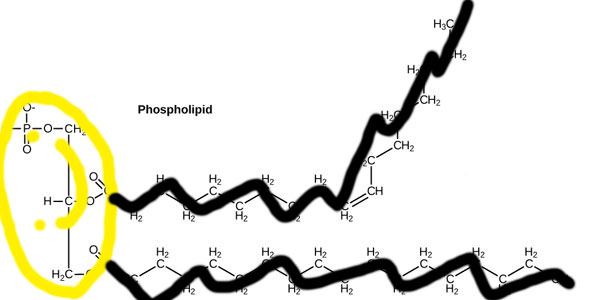
There, that looks much less scary and easier to comprehend. Or does it?
Source: OpenStax, Concepts of Biology, modified.
So what's gonna happen? Well, remember that scene in the movie Stuck on You where conjoined twin and womanizer Greg Kinnear was having sex with a woman he just picked up while his more sensitive conjoined brother Matt Damon was sending a romantic text to a love interest? That's more or less what would happen. But let's go into some more detail, because this is the gist of the article.
How membranes form spontaneously
Now this is the part that gets my heart pumping. I'm serious. This is the stuff I'd share around campfires while others share their ghost stories. Hey, my story might make some people sleepy, but at least it's true!
When you throw in an amphipathic molecule that has both a hydrophilic (water-loving) part and a hydrophobic (water-fearing) part into water, there's gonna be a problem. There's three ways that this problem can be resolved. In other words, there's three arrangements that the molecules can adopt that will resolve the tension. Way number one:
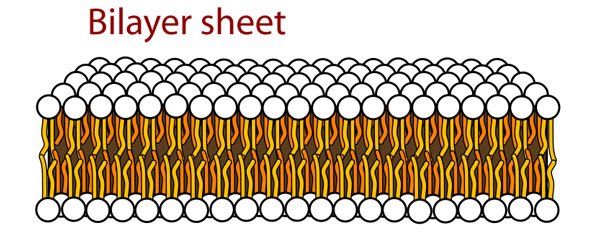
Source: Wikimedia Commons contributors, modified.
Hey, I know these guys! It's our good ol' friends the smileys with their tails! Arranged in a bilayer sheet configuration! That will keep their hydrophobic tails inside, leaving their hydrophilic heads to interact with the water surrounding them all they want! That's some smart thinking. Or, it would be, if it didn't happen spontaneously. Let's move on to way number two:
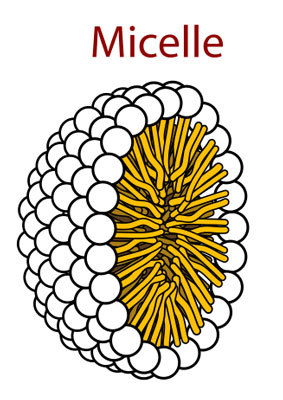
Source: Wikimedia Commons contributors, modified.
Hey, what do we got here! The amphipathic molecules have decided to take on a round shape. This is slowly starting to look like a regular cell. Hence the name I guess. Can't wait to see what comes next!
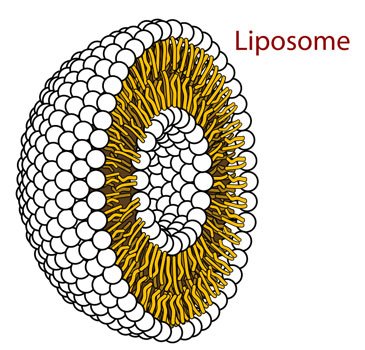
Source: Wikimedia Commons contributors, modified.
Dun dun dun! Enter the liposome. I can already imagine a nucleus housing a DNA molecule in that waterhole in the middle. Smart houses ain't got nothing on this. And what did we do to make it happen? Nothing: we just threw in some amphipathic molecules in water and shook them around. And I just added that last step for flourish: it's not really necessary.
So there you got it folks, the story of how membranes are made spontaneously. Obviously the cells that make you up are a little bit more involved, more like this:
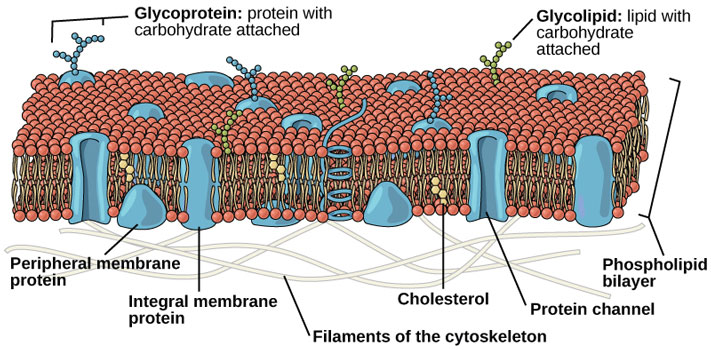
Source: OpenStax, Concepts of Biology, modified.
But you can still see our friends the smiley faces with tails playing a center role in there. To my eye, they're the most abundant thing in that picture.
Curtain close
In the previous "introduction to biology" post I examined vitalism, the idea that life was something beyond just chemistry and physics, and I showed the Nobel Prize experiment that blew that theory out of the yeast water. In this post, in keeping with that same mechanical approach to life, I explained how cell membranes form spontaneously simply because of the laws of chemistry. You don't need no angel dust!
I find it's easier to learn any subject, when we somehow relate it to our human inquiries and interests. Biology is not just about memorizing data. It's about understanding how we came to be here. It's about making sense of the world. And there's no better place to start — or end — than our cells.
And now we can go out there and hug people with not a worry in the world that we'll spill into each other!
References:
Wikipedia contributors, "Human skin," Wikipedia, The Free Encyclopedia, https://en.wikipedia.org/w/index.php?title=Human_skin&oldid=808028388 (accessed November 4, 2017).
OpenStax, Concepts of Biology. OpenStax CNX. Jul 17, 2017 http://cnx.org/contents/[email protected].
Molecular Biology of the Cell. 4th edition. https://www.ncbi.nlm.nih.gov/books/NBK26883/
Wikipedia contributors, "Lipid bilayer," Wikipedia, The Free Encyclopedia, https://en.wikipedia.org/w/index.php?title=Lipid_bilayer&oldid=800307900 (accessed November 4, 2017).
steemSTEM is the go-to place for science on Steemit. Check it out at @steemstem or browse the #steemSTEM tag or chat live at steemit.chat
Life, like ideas and cultural matrix, require specifed, defined boundaries to exist. In the modern era of "tolerance" and "multiculturalism," reflecting upon the fundamental biological unit seems relevant to current social condition.
There is, of course, the division between prokaryote and eukaryote cells, whose biological matrix differ in significant fashion that antibiotics selectively kill prokaryotes and spare eukaryotes. There are exponentially more prokayote cells residing within the matrix of the collection of eukaryote cells we call human. In some sense, a human is but a carrier platform for the trillions of prokaryote cells; a fact upon which a man may wish to reflect, whenever he is infected with hubris to perceive himself a god.
Indeed there's quite a lot of parallels one can draw between what cells (or micro life) do and how they interact, and humans (or macro life).
Junk DNA has fueled quite a lot of speculation and even sci fi stories. An idea about a short story I had many years ago, but never got to write it, was humans as a kind of walking USB stick coding a message for aliens. But that sounds like the kind of thing someone must've thought of by now.
But the term "junk" isn't used by biologists, who prefer to use the word transposons. And rightly so: "junk" implies there's no use to them, when there might well be. For example, because transposons are mutagens (they hop around our DNA randomly, which means they may hop into a coding DNA region, causing a mutation) some say transposons can be a driver of evolution.
DNA may be like computer codes (binary 0s and 1s, except it is quartenary ACTG coding). Back in the days of IBM 384s and Apple IIs, the computing power was enormously underwhelming that programming codes were always elegantly stripped of unnecessary entries. In this era of cheap processing power, codes have become cumbersome and obtuse. It is understandable evolution, since time expended streamlining codes can be used elsewhere, now processing power of these computers make elegance irrelevant.
I wonder if prokayote plasmids are littered with "junk" DNA, as eukaryote DNA. Mitochondria and endoplasmic riticula have made elegant coding irrelevant in eukaryotes, much like IBM Big Blue have made streamlining a waste of time.
Cell biology is so insanely complicated. Yet, looked at another way, it seems inevitable.
In any case, the more I read about how our bodies work, the more amazed I am that they do work, at all.
In one sense I agree. But in another sense, the fact that we can understand cell biology at all, tells me maybe it's not that complicated, objectively speaking: it's just our brains aren't that great :P
Think of it this way: About 4 billion years ago Earth cooled, oceans formed, so life was made possible. Only about 300 million years after life was made possible, maybe even earlier, the first life (prokaryotic) appeared. See here (I love the phrase "an almost instantaneous emergence of life").
Note that the first euks appeared ~3 billion years after that. You heard right: 300 million years for life to appear, vs 3 billion for proks to become euks. I'm oversimplifying, but euks are just proks with nuclei. I.e. basically more of the same: a membrane within a membrane.
Lots of numbers are thrown out there in the form of statistics, but the facts tell me it's not that difficult to make life (so it shouldn't be that complicated).
Initially I wanted to write a post about how easy (or hard) it is to make life, but decided against it due to the scope, and zoomed in on a particular detail instead: how cell membranes spontaneously form due to the laws of chemistry.
Gag... :)
:P
A very seriousl post and the way it describes it makes it understandable. the truth is that the human body works with electrochemical reactions and its main reason for being is water and the carbon, but it does not cease to be a miracle of nature! :)
This is an excellent post I am so impressed thank you for sharing with us.
@OriginalWorks
The @OriginalWorks bot has determined this post by @alexander.alexis to be original material and upvoted(1.5%) it!
To call @OriginalWorks, simply reply to any post with @originalworks or !originalworks in your message!
Congratulations @alexander.alexis, this post is the third most rewarded post (based on pending payouts) in the last 12 hours written by a User account holder (accounts that hold between 0.1 and 1.0 Mega Vests). The total number of posts by User account holders during this period was 2168 and the total pending payments to posts in this category was $2556.99. To see the full list of highest paid posts across all accounts categories, click here.
If you do not wish to receive these messages in future, please reply stop to this comment.
Congratulations @alexander.alexis! You have completed some achievement on Steemit and have been rewarded with new badge(s) :
Click on any badge to view your own Board of Honor on SteemitBoard.
For more information about SteemitBoard, click here
If you no longer want to receive notifications, reply to this comment with the word
STOPI recall wondering, long ago, how it is water (as a body) exists. In other words, I understand what makes a water molecule, but I do not recall ever seeing an explanation as to why these molecules stay together to form a body (should I say, a discernable body) or mass of water for slaking my thirst. I can imagine that trying to satisfy my thirst, molecule by molecule, would not be very satsfying.
Thanks, I've learnt a bit from you, you are much better at it than my teachers ever were (more than 60 years ago).
:)
This comment somehow fell by the wayside!
Yes, the water(s) are hydrophilic, so they like to interact with themselves, which is why they form beads on surfaces, instead of being flat. The bonding is so strong that bugs can walk on water, aptly called Jesus bugs!