Otto Cycle
Otto cycle
The Otto cycle is the thermodynamic cycle that is applied in the internal combustion engines of ignition caused (gasoline engines). Invented by Nicolaus Otto in 1876. It is characterized because in a first theoretical approach, all the heat is contributed to constant volume
Diagram of a 4-stroke Otto cycle in a PV diagram
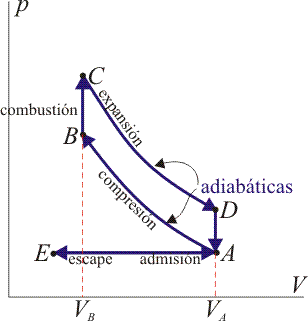
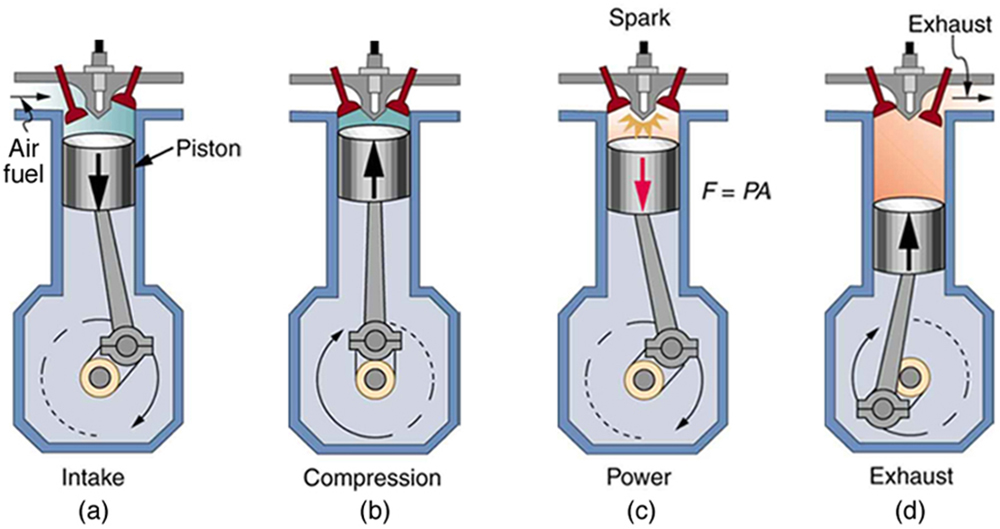
Stages of an Otto cycle
An ideal Otto cycle is a theoretical approximation to the behavior of an internal combustion engine. The phases of operation of this engine are the following: Admission (1), Compression (2) Combustion Expansion (3), Exhaust (4)
AdmissionThe piston goes down with the intake valve open, increasing the amount of mixture (air + fuel) in the chamber. This is modeled as an expansion at constant pressure (since when the valve is open the pressure is equal to the outside). In the previous diagram shown PV appears as the straight line E → A.
Compression
The piston goes up compressing the mixture. Given the speed of the process, it is assumed that the mixture has no possibility of exchanging heat with the environment, so the process is adiabatic. It is modeled as the reversible adiabatic curve A → B, although in reality it is not because of the presence of irreversible factors such as friction
Combustion
With the piston at its highest point, the spark from the spark plug jumps. The heat generated in combustion abruptly heats the air, which increases its temperature to practically constant volume (since the piston has not had time to lower). This is represented by an isoquator B → C. This step is clearly irreversible, but in the case of an isocular process in an ideal gas the balance is the same as in a reversible one.
Expansion
The high temperature of the gas pushes the piston down, doing work on it. Again, because it is a very fast process, it is approached by a reversible adiabatic curve C → D.
Escape
The exhaust valve opens and the gas exits to the outside, pushed by the piston at a temperature higher than the initial temperature, being replaced by the same amount of cold mixture at the next intake. The system is really open, because it exchanges mass with the outside. However, given that the amount of air that comes out and that enters is the same, we can assume that the air is the same, that has cooled down, for the energy balance. This cooling occurs in two phases. When the piston is at its lowest point, the volume remains approximately constant and we have the isotope D → A. When the piston pushes the air to the outside, with the valve open, we use the isobar A → E, closing the cycle.
In total, the cycle consists of two rises and two drops of the piston, which is why it is called a four-stroke engine.
In a real engine of explosion several cylinders act simultaneously, so that the expansion of some of them performs the work of compression of others.
INFORMATION SOURCES
(image 1 )http://laplace.us.es/wiki/index.php/Ciclo_Otto
Congratulations @gerardoalfred! You have completed some achievement on Steemit and have been rewarded with new badge(s) :
Click on any badge to view your own Board of Honor on SteemitBoard.
For more information about SteemitBoard, click here
If you no longer want to receive notifications, reply to this comment with the word
STOP