Nucleation process in crystallization technology;polymorph preparation.#4
Hi everyone,I hope you are enjoying your day? I guess you do. Before I continue with different means of obtaining polymorphs in pharmaceutical industry,I want us to check something very vital.
We need to know the importance of polymorphism in industries.Mind you, polymorphism is a phenomenal not only peculiar to pharmaceutical industry alone but also in pigment , agrochemical, explosives and electrical industries too.
The two main importance are;
- Polymorphism existence in solid materials is inevitable.What that means is that polymorphism is a phenomenal that happens during discovery, invention and manufacturing.
According to Ostwarld
Almost every substance can exist in two or more solid phases provided the experimental conditions are suitable.
Source
2 The second reason is for an intentional purpose.This means you wanted it to happen.Let me say one can intentionally change the physicochemical properties of compound under study by using more than one polymorphs.Like I said earlier, that polymorphism is inevitable and can hardly be avoided in drug formulation, it's understansting is as important as the drug.Many drugs that claimed not to exhibit polymorphism before,had eventually agreed to the phenomenal, because of the advance in research that disprove their claim.
Let's get to business now by looking at how to prepare or obtain polymorphs in drug formulation. It should be noted that different condition during crystallization process is the fundamental reason for various polymorphic form development.
Let me start off with number on my list here.
Crystallization using one or mixed solvent.
Generally, screening of polymorphs can be done by Crystallizing materials from one or mixed solvent through cooling crystallization, evaporating the solvent, or using antisolvent crystalization.
A major challenging factor in using this method is solvent selection.More than 96 well known solvent are available and can be used for polymorphs development and screening.
These solvents for polymorphs have been classified into fifteen categories with the aid of cluster statistical analysis. Properties of solvents such as hydrogen- bond acceptor or donor tendency, solvent polarity, solvent dipole moment, it's electric constant and many more are variable. Solvent selection can be made from each group for primary polymorph
evaluation and mix solvent various solvent from different groups.
Other factors to be considered in addition to solvents selection include heating and rate of cooling, temperature at which crystallization occur, rate of evaporation, level of supersaturation, rate or pace of agitation, media pH are all variable which can determine the Crystallization process and type of formed polymorphs.
Thermally activating solid substrate.
Thermodynamic relationship between polymorphs is highly important to be understood before thermally activating solid substrate.
As I explained earlier in the previous post that polymorphs could either be monotropic or enantiotropic system.It should be noted that any two given polymorphs can be either of the two system. Monotropic system occur when a polymorph is stable or established over entire range of temperature. For the enantiotropic system,one state of the polymorph is stable below transition temperature, while the other form is established above the transition temperature.
The metastable form at room temperature in an enantiotropic system is obtainable when the stable form which is above the transition temperature is heated.
While the stable form at room temperature in a monotropic system is gotten when the metastable form at any temperature is heated.
Transformation rate using this method can be made easier and faster by heating the metastable form at high temperature.To obtain metastable form through thermal activation mechanism in a monotropic system might be difficult if the substrate used is in it stable form.
An example is flufenamic acid I which is gotten when flufenamic acid III is subjected to heat above 105°C.The metastable form at room temperature of the polymorph which is flufenamic acid I is obtained.
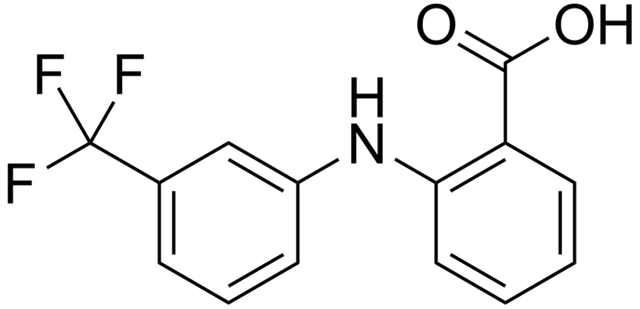
Flufenamic acidBy Edgar181 - Own work, Public Domain, https://commons.wikimedia.org/w/index.php?curid=2868074
One can estimate the transition temperature by observing the occurrence of transition throughout DSC measurement, by determining through calculation the Gibbs free energy variance between polymorphs over the range of temperature through directly taking the measurement of heat capacity by drawing the van Hoff's plot en route solubility determination of polymorphs over temperature ranges possible, or by making the mixture of the polymorphs slurry over temperature available
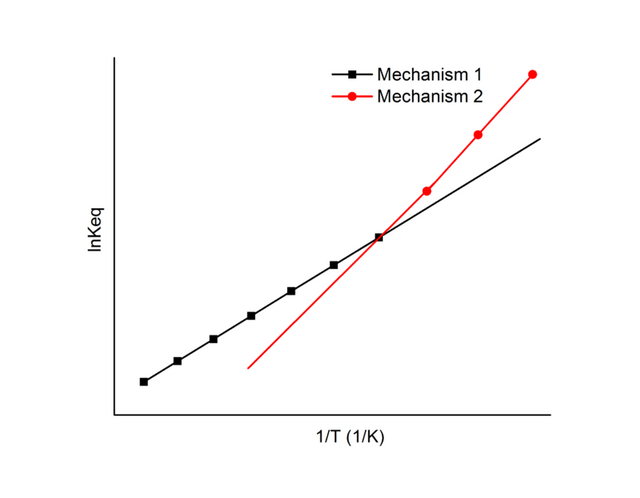
van Hoff's plot By CarboJoule - Own work, CC BY-SA 3.0, https://commons.wikimedia.org/w/index.php?curid=29092876
Crystallizing through the melt.
Method of Crystallizing through the melt is quite similar to Crystallizing from amorphous material. Because amorphous materials (they are any non crystalline materials that it's component atoms or ions or molecules does not have a definite lattice structure or pattern) are thermodynamically unsteady form when relatively compared with crystalline materials, amorphous substances gravitate to crystallize.
Actually to crystallize from amorphous material maybe a bit difficult because amorphous modify the physicochemical properties of a drug.One advantage is that crystallization from amorphous can be use as a technique to develop required polymorphic form.
nifedipineBy Ayacop - Own work, Public Domain, https://commons.wikimedia.org/w/index.php?curid=1524529
The external tension on amorphous determine the type of polymorphic form that will be generated from the crystallization of the melt with different kinetics and process.
Crystallization through the melt has been severally reported to be the only means of obtaining the metastable form of a drug compound called nifedipine.The two metastable forms of the drug are II or beta and III or gamma forms.Its also use to obtain metastable form III of paracetamol known as acetaminophen.
Acetaminophen skeletal also known as parecetamol By Benjah-bmm27 - Own work, Public Domain, https://commons.wikimedia.org/w/index.php?curid=826823
Seeding
Seeding promote crystallization through secondary or heterogeneous nucleation. Industrial level of crystalization required a technique that will give a desired polymorphic form. Seeding as a means provide this platform.
Pseudoseeding can be employed when the desired seed is not available.It is forecasted that the polymorphic form of the seed will be obtained.It should be noted that seeding technique can aid the Crystallizing of some unwanted polymorphic forms when those seeds used serves as model for cross nucleation.When such occur, the polymorph that grow faster than the seed crystal will be obtained as crystal.
Crystallization in nano confined pattern.
Nano-sized pores can be used to grow crystals, this helps to alter the relative thermodynamic steadiness between polymorphs and to boost the physical firmness of the metastable polymorph just as we have it in paracetamol known as acetaminophen form III to have remotely known polymorphs such as sigma pimelic acid, beta suberic acid and beta-coumarin or Crystallizing the stable forms such as mefenamic acid, glycine and ROY that are difficult to be formed under right method of crystallization.

Suberic acid By Emeldir (talk) - Own work, Public Domain, https://commons.wikimedia.org/w/index.php?curid=31383845
Desolvation or Dehydration of Solvate /hydrate or Reslurry.
There is a tremendous increase in the number of solvates/hydrate noticed when screening process is taking place.This is observed as there is an increase in the experiment number during the polymorphic screening process.
Solvates undergo phase change during dehydration which eventually leads to the formation of non-solvated or anhydrous polymorph or it may leads to formation of non-crystalline solid.
In some instances, solvent may leave the crystals having less impact on the solvate structure of the crystal.
Mostly, desolvation process is carried out at low relative humidity or at times low organic vapour pressure.
Also, relevant polymorphs have being obtained when desolvation using reslurry solvates in a solvent with poor solubility at either low or high temperature method is applied.
Slurry conversation procedure.
Polymorphic transformation mediated in solution is a quick and reliable means to produce steady polymorph.If all condition for this method is meet, it is possible to obtain polymorphs that follows Ostwald's rule of stage.
This method take place by dissolution of the metastable form which is then followed by nucleation of another polymorph which is more stable than the initial form.
The kinetic influence regulate the rate of solution-mediated polymorphic transformation with the difference in solubility between polymorphs in a given solvent inclusive, and rate of dissolution in the metastable form, and the level of nucleation of the stable form. It is highly important too to note that particles size play a vital role in deciding polymorphic transformation mediated in solution rate.
The method is also a very easy and simple means to obtain solvates of a compound. In a very broad sense, solvates or hydrate is the most stable state in a particular solvent if the compound of interest show solvate forming inclination.
If some particular solvates or hydrate, are required, crystals devoid of solvent can be slurried in the used solvent for an elongated period of time.For instance if two polymorphs free of solvent are taken for consideration, the stability of the thermodynamics of the two polymorphs can only be ascertain by difference in Gibbs free energy at a specific temperature and pressure.
If hydrate/anhydrous polymorph form are in consideration, activity of water in the solvent contributes to the relative physical stability of such and the polymorph obtained as a result of that.Water activity taking place in a solvent mixture have a great impact in determining the form of crystal obtained.This is regardless of the organic solvent used.
Summary
In this post, I discussed importance of polymorphism in industry generally as it is not only peculiar to pharmaceutical industry alone.I also discussed about seven methods of obtaining polymorphs in pharmaceutical industry.
Note that all images are from free source.Thanks
Image source
All image source are embedded in the image name.
This is a detailed topic @steepup, well done and keep it up.
Thanks @noble-noah.I appreciate you stopping by.
Though the image sources are embedded, you are merely providing a link to a bigger version of the same image. This is not a source, per se, nor is it crediting any original author. Please make sure to provide a link to the actual source, and make sure they are available for commercial use.
Good luck!
Thanks very much corrections made.
As a follower of @followforupvotes this post has been randomly selected and upvoted! Enjoy your upvote and have a great day!
Congratulations! This post has been upvoted from the communal account, @minnowsupport, by Steepup from the Minnow Support Project. It's a witness project run by aggroed, ausbitbank, teamsteem, theprophet0, someguy123, neoxian, followbtcnews, and netuoso. The goal is to help Steemit grow by supporting Minnows. Please find us at the Peace, Abundance, and Liberty Network (PALnet) Discord Channel. It's a completely public and open space to all members of the Steemit community who voluntarily choose to be there.
If you would like to delegate to the Minnow Support Project you can do so by clicking on the following links: 50SP, 100SP, 250SP, 500SP, 1000SP, 5000SP.
Be sure to leave at least 50SP undelegated on your account.