CARBON MONOXIDE AND OUR HEALTH…

image source
Hello guys I am here once again with yet another interesting topic. One that concerns us. One that relates to man and his environment. One that we should all be wary about.
Today I will be writing extensively on the topic carbon monoxide and how it relates to our health . I am sorry if those who are not science inclined can’t relate, but as usual, I will try as much as possible to break terms and explanations down so that every single person reading this article will relate and learn.
For those who have no idea what carbon monoxide is, but hear about it on a daily basis. It is that gas that comes out of a car or generator exhaust pipe or that gas that is emitted from a chimney or from an industrial exhaust pipe.

image source: carbon monoxide from a car exhaust pipe
Carbon monoxide is produced as a result of the incomplete combustion/burning of natural gas (fuel) and other materials containing carbon such as gasoline, kerosin, natural oils, propane, coal or wood. Forges, blast furnace and coke ovens also produce carbon monoxide. It is more like a waste product but in this case, very harmful to humans and animals when inhaled.
Carbon monoxide (CO) is a poisonous, colorless, odorless, and tasteless gas. But many of you would wonder why the fumes that come out of the car exhaust pipe or our domestic power generators smell aweful. You will be asking an intelligent question if you do and I will tell you why it’s so.
You see, although carbon monoxide has no detectable odour or smell, it is often mixed with other gases that do have an odour. So, you can inhale carbon monoxide right along with gases that you can smell and not even know that carbon monoxide is present.
Since now know what carbon monoxide is in clear and understandable terms, let us now take a look at how carbon monoxide came to discovery in the history of carbon monoxide.
HOW CARBON MONOXIDE WAS DISCORVERED…
Aristotle, a Greek philosopher and scientist, first recorded that burning coals produced toxic fumes but at the time he didn’t know what those fumes where.
Back then, an ancient method of executing criminals was to shut the criminals in a bathing room with smouldering coal (by smouldering coal, I mean coal that is about going off and so still releases some kind of fumes know presently as carbon monoxide…it’s these fumes that actually make smoked fishes taste the way they do).
Now at that time, what was not known to those who practiced this act of execution was the mechanism of death. They didn’t know what actually killed these criminals but all they knew was that it was a very effective method of execution.
Between 129-199AD, a Greek physician called Galen speculated that there was a change in the composition of air that actually caused the deaths, but hey, it was just a speculation and so didn’t hold water at that time until a French chemist, in 1776, called De Lassone produced the carbon monoxide gas by heating zinc oxide with coke (please coke is a form of carbon and not coca cola…lolzz). Coke is a solid fuel made by heating coal in the absence of air and by so doing, all the volatile components are driven off while zinc oxide is an inorganic salt which is white and insoluble in water.
Though De Lassone mistakenly concluded that the gaseous product was hydrogen because it burned with a blue flame. However, in 1800, a Scottish chemist, William Cumberland Cruikshank, finally identified the gas as a compound containing Carbon and Oxygen (CO) and that was how carbon monoxide gas was discovered.
Having come to the foreknowledge of the discovery of carbon monoxide, lets now take a look at the physical and chemical properties of carbon monoxide.
Below is a table showing the physical and chemical properties of carbon monoxide in simplified terms
| PROPERTY | CARBON MONOXIDE |
|---|---|
| Molecular weight | 28.01 |
| Chemical formula | CO |
| Chemical structure | :C≡O: |
| Colour | colourless |
| Physical state | gas |
| Melting point | -205 oC |
| Boiling point | -191.5 oC |
| Density at room temperature (25 oC) | 1.145 g/L at 25 °C and 1 atm |
| Odour | Odourless |
| Solubility in water at 20 oC | 2.3 mL/100 mL |
| Solubility In organic solvents | Appreciably soluble in ethyl acetate, chloroform, and acetic acid; freely absorbed by a concentrated solution of cuprous chloride in hydrochloric acid or ammonium hydroxide; solubility in methanol and ethanol about 7 times as great as in water; soluble in benzene. |
| Flashpoint | Flammable gas |
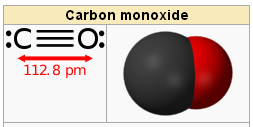
image source: Chemical structure of carbon monoxide gas
Now that we know the physical and chemical properties of carbon monoxide, let us now see how this poisonous gas affect us when we inhale it.
How does carbon monoxide affect our body upon inhalation?
Carbon monoxide is harmful when inhaled because it displaces oxygen in the blood and deprives the heart, brain, and other vital organs of oxygen. Large amounts of carbon monoxide can overcome you in minutes without warning, causing you to lose consciousness and suffocate.
Besides tightness across the chest, initial symptoms of carbon monoxide poisoning may include headache, fatigue, dizziness, drowsiness, or nausea. Sudden chest pain may occur in people with angina.
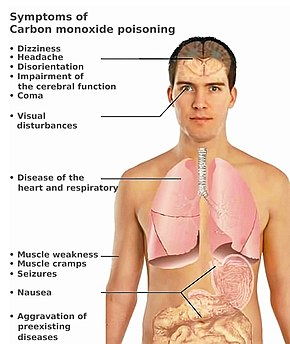
image source: Symptoms of carbon monoxide poisoning
During prolonged or high exposures, symptoms may worsen and include vomiting, confusion, and collapse in addition to loss of consciousness and muscle weakness. Symptoms vary widely from person to person.
Carbon monoxide poisoning may occur sooner in those most susceptible: young children, elderly people, people with lung or heart disease, people at high altitudes, or those who already have elevated carbon monoxide blood levels, such as smokers.
Also, carbon monoxide poisoning poses a special risk to foetuses (undeveloped babies). Carbon monoxide poisoning can be reversed if caught in time. But even if you recover, acute poisoning may result in permanent damage to the parts of your body that require a lot of oxygen such as the heart and brain which could eventually lead to stroke.
If you are exposed to carbon monoxide over a long period of time, you can still be poisoned, even if the level of concentration is very low as seen with cases where people put off their generator sets and carry it into their house and by the next morning we see the whole family being pronounced dead. Lesser concentrations of 20 or 30 PPM (parts per million) can still be harmful if you are exposed for several hours. It is not known whether lower and shorter levels of exposure can cause permanent brain damage, however, they are likely to cause persistent headaches, memory loss, depression, light-headedness, nausea and vomiting.
What happens to the respiratory system when someone inhales carbon monoxide?
The respiratory system struggles to distribute air around the body because carbon monoxide deprives the blood cells of oxygen. This results in shortness of breath, particularly when undertaking strenuous activities. Every-day physical and sporting activities will take more effort and leave you feeling more exhausted than usual. These effects can worsen over time as your body's power to obtain oxygen becomes increasingly compromised.
Both your heart and lungs are put under pressure as the levels of carbon monoxide increase in the body tissues. The heart will try harder to pump what it wrongly perceives to be oxygenated blood from your lungs to the rest of your body. As a result, the airways begin to swell causing even less air to enter the lungs. With long-term exposure, the lung tissue is eventually destroyed, resulting in cardiovascular problems and lung disease

image source
Conclusion...
So from all these, we can see that carbon monoxide is very dangerous to our health and must be avoided like a plague. One cannot totally avoid inhaling carbon monoxide but try as much as possible not to get exposed for a long time.
Congratulations @sistem! You have completed some achievement on Steemit and have been rewarded with new badge(s) :
Click on any badge to view your own Board of Honor on SteemitBoard.
For more information about SteemitBoard, click here
If you no longer want to receive notifications, reply to this comment with the word
STOPJust awesome post regarding CO. It covers almost all the aspect of it.
By the way, it is really dangerous gas and can kill you if the concentration of it in inhaled gas is high. So need to be careful. #air-clinic