HOW TO RECOVER SAMPLES AND SUBSTANCES IN THE LABORATORY
Good day, my dear readers and welcome to another episode on the basic techniques used in the laboratory.
This post is as a result of my past experience on the different method on how to recover the substance (Chemistry Practical), the instructor told us during a class work that, Recovery of substances in the laboratory is a very important process that enables the substance to separated from each other,
Then arose the question, why does it need to separate the samples?. Does the samples easily separated?. What is the basic principle or techniques behind this? Please don't border to ask me if I gave into the temptation because you can't imagine what happened on this fateful day.
Recovery of substances in the laboratory is a very important process and can generally be discussed under the following sub-headings.
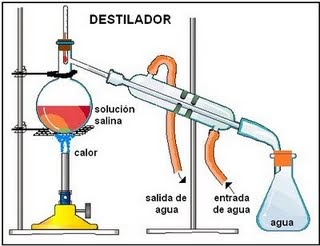
Fractional Distillation
Fractional distillation can be used to separate miscible liquids such as water and ethanol. The process involves the collection of fractions at different temperatures corresponding to the boiling points of the liquids.
Materials required for fractional distillation in the laboratory are as follow,
- Bunsen burner,
- A round-bottomed flask,
- A condenser (water in and out)
- and fractionating column,
- Thermometer.
Industrial Fractional distillation: This is a technique used to separate different hydrocarbon in petrochemical, in petroleum refineries and chemical plants. The fractional tower or distillation or distillation columns, which is ranging from 65 centimeters to 6meters and height are from 6 to 60 meters. The outlets of liquid distillation towers have the intervals on the column that permit the different fractions or products withdrawal of having different boiling ranges and boiling points.
For example, The fractional distillation used in oil refineries, to separate crude oil into useful substances and having different hydrocarbons of different boiling points and the crude oil fractions with higher boiling points:
Using Separate Funnel: A separating funnel can be used for separating immiscible liquids (liquids that are not soluble in one another) such as kerosene and water.
How does the separating funnel work?
Separating of solvent or liquid in the laboratory is carried out by using a separating funnel and the items to separate must have been mixed together and left for sometimes, it’s must form some different layers before the separation take place
What is decantation, Filtration and Sedimentation?
Decantation And Filtration: Decantation is usually adopted to separate solids from liquids by allowing the solids to settle to the bottom of the container and then pouring off the liquid. This method is very good for separating a heterogeneous mixture of a solid and a liquid where the solid, being denser, settles to the bottom of the container.
Decantation method can be used to separate completely a liquid from a solid subject to the solid being able to completely settling down to the bottom. Where the solid remains in suspension with the liquid, filtration is better adapted.
Filtration is the process of separating a solid from a liquid by allowing the mixture to pass through a filter. It is, however, subject to certain variables such as the physical nature of the mixture, that is, volume; viscosity and volatility of the liquid. The amount of the solid present and its particle size. To make filtration more effective, it is better performed under pressure. Sedimentation is the process whereby the settling down of heavly particles take place in the experiment.
What is recrystallization?
This is a method of purification that is suitable for separating crystalline solid. The method which depends on the principle of solubility is widely adopted in purifying solids. This method involves dissolving the substance (to be crystallized) in a solvent in which it is highly soluble at the boiling point of the solvent but which will begin to crystallize out as the temperature drops.
What is recrystallization is used for?
In the separation of sample and solvent, recrystallization is the technique be applied to purify chemicals, by the ways dissolving the compound in a certain amount of solvent and impurities, maybe the mixture of sample and impurities can be separated from the solution.
What is the difference between recrystallization and crystallization?
When the conditions warrant, where the materials are no more soluble, then it can’t be used or obtainable as purity again. Recrystallization is the method or techniques used to purify solid substance into a solution and modified the conditions to allow the crystals to change the position (reform).
What is the purpose of the recrystallization process?
The main purpose of the recrystallization process is that normal technique must applied so as to purify the solids substance. When impurity samples mixed with two or more components, one of the component are the impurity while other components are desired.
In this case, solubility can be said to vary directly with the temperature, i.e., increase in temperature will lead to increase in solubility and vice- versa. This method has been widely used in the purification of drugs especially the analgesics and the antipyretics e.g Aspirin.
Distillation
This process is suitable for separating the liquids with varying boiling points. The process has been widely adopted in the production of spirits and other related drinks. It involves heating the mixture of alcohol and water to boil while each of the substances separates out bat their various boiling points, i.e., alcohol - 78C and water - 100C. Related to this technique is fractional distillation adopted in petroleum refineries to separate crude oil into its various fractions.
Centrifuigation
This is a method usually adopted for separating a lighter portion of a mixture, solution, and suspension from a heaver portion through the application of a centrifugal force.
It involves the use of a centrifuge to hasten the disposition of substances suspended in liquids. In the end, the suspended substances settle down to the bottom of the container in order of weight with the heaviest being the first to settle.
Why we do centrifuge?
The centrifuge is an equipment or machine used for spinning the samples in the laboratory at required speeds and the percentage of the samples drop into test tubes and subjected to centrifugal force, the dense particles move away from the axis of rotational force and the other parts move toward it, for the purpose of research or investigation.
What is centrifuged blood?
A blood plasma that is clear solution in the upper phase (which led to the separation of blood plasma) into its fractions, then the thin layer of leukocytes (white blood cells) mixed with platelets in the middle, and erythrocytes (red blood cells) at the bottom of the centrifuge tube.
IMAGE CREDIT
image 1
REFERENCE
ref 1



The google search function does not always indicate if an image is ok for reuse. For example, eventhough you searched for this image under labeled for reuse it indicates below the image that google is not sure if it is copy right protected. You need to find an explicit mention on the website of the image that it has the right copyrights for reuse.
Well done @olayiwola, you have taken me back to my secondary school days, where separation techniques is an integral part of chemistry.
Please, try to relate more with SteemStem community, am sure you are on discord.

Kind regards.
@noble-noah,thanks you sir, more of this post is still coming.
I find this interesting. I have almost forgotten my high school chemistry :)
Hmmm nicely done
I love this refreshing post, it made me remembered my high school days and high school exams (waec).
Keep steeming. Thumbs up
Congratulations! You have been upvoted by Grayesque. Your post will be featured on our daily curation posts.
Our curation team is currently formed by @eddy-18, @jo5h, @chinyerevivian, @biblio and @seesladen. We scout Steemit in search of excellent posts deserving more exposure and we believe your post is one of them.
Hehe...... This post drew me closer to my modern chemistry textbook.
Well explained process of separation. I guess I learnt some new techniques.
Lovely article
Haha, chemistry tho. This very topic will always stick.
I was immediately thrown aback to my high school days..
Thanks for this compilation
I had a powerful cabinet with a pump for this, so that all the vapors would evaporate. I bought laboratory fume hoods at the store https://topairsystems.com/. The equipment is used for conducting experiments, analyses and research. Suitable for industrial enterprises, educational institutions, individual laboratories. Its peculiarity is the presence of a hood that absorbs dangerous volatile substances. This ensures safe operation and accurate results. Laboratory cabinets with ventilation may vary in design, dimensions, materials.