Graphene: A real life Vibranium?
Your pencils are made of graphite, probably you’d be saying ‘I’m already aware of this of course ‘, that’s almost certain. However, very peculiar to you also is the marvelous black colour your pencil makes when you drag it along your note books or drawing boards, making constructive drawings and shadings as the case may be, but actually you were close to winning a Nobel Prize, if only you had thought about shedding the dark marks made by your pencil to a thickness of one atom—that’s graphene you would have discovered, but it’s already late.
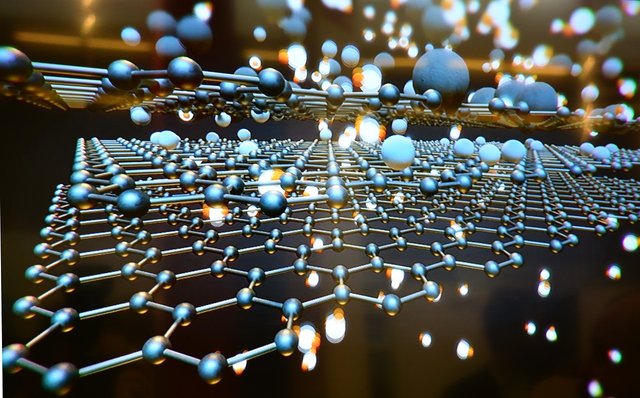
Structure of graphene image source~pixabay. CCO creative commons
Graphene has been a subject of scientific research for years, it had been produced in minute quantities for centuries, through the use of pencils and other graphite containing substances , it was originally observed in electron microscopes in 1962,but it was studied only while supported on metal surfaces, the graphene was later rediscovered, studied and characterized in 2004 by Andre Geim and Konstantin Novoselov at the University of Manchester. The two eventually won a Nobel Prize in physics in 2010 for ground breaking experiment regarding the wonder compound ‘graphene’. As we blow off the excess pebbles from our pencils, the tinniest of them was probably a two dimensional allotrope of carbon—graphene.
If you are a fan of sci-fi movies, you must have watched the very popular marvel entertainment 2018 movie ‘Black Panther ’,if yes, then you must have a handy knowledge about the ‘Vibranium’ a very wonderful fictional metal which formed the bulk of the technology, culture and lifestyle of the fictional African nation of Wakanda, so powerful as it was, the vibranium could permeate plants and animals found in this nation, giving anyone who takes it a super human abilities, the vibranium is however a fictional wonder element of and was used in producing the black Panther’s suit which is able to absorb kinetic energy within its bonds, however, that was just for entertainment as such metal is yet to be discovered. But maybe we’ve got our own kind of vibranium in this awesome allotrope of carbon, the ‘graphene’.
Meet ‘Carbon'; The mother figure.
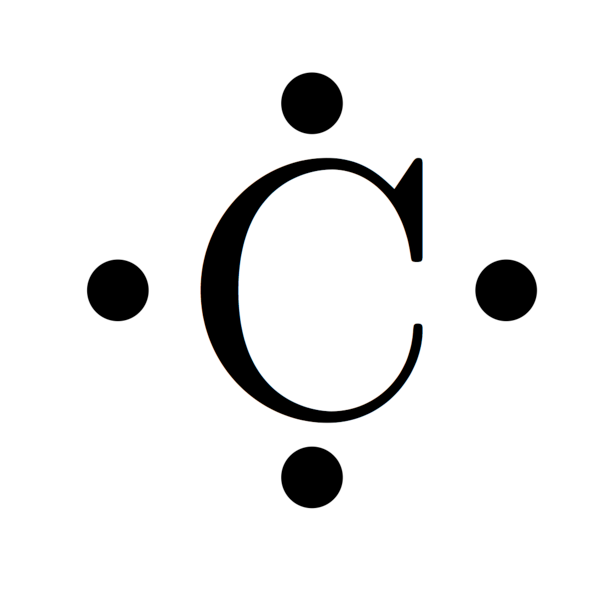
Lewis carbon structure wikimedia commons license Creative Commons Attribution-Share Alike 4.0 International
Carbon, the fourth most abundant element in the universe by mass is a tetravalent non-metal, with four (4) electrons available for the formation of covalent bonds, carbon forms a vast number of compounds more than any other element with about 10 million carbon containing compounds known till date, many things in our ecosystem contains carbon, from limestones and dolomites to the carbon dioxide we exhale and carbon monoxide from our car exhaust pipes.
Carbon forms a bulk of organic matter and the fossil fuels, in metallurgical industries, carbon is alloyed with iron to form steel, to mention but a few, the appreciable reactive abilities of carbon makes it a valuable element and since it’s first discovery in 3750BC, more discoveries have been made about carbon and of importance is the ability of carbon atoms to bond with themselves in different ways to form compounds of varying physical properties but basically containing carbon, this phenomenon is known as Allotropy in chemistry, apart from carbon, many other elements such as sulphur have been found to exhibit allotropy, this is mainly due to their valency, presently, over 500 hypothetical 3-periodic allotropes of carbon are known.
A shallow look Into the most important allotropes of carbon will be necessary for the comprehension of other parts of this article.
Allotropes of carbon
Diamond

Diamond is one of the major allotropes of carbon. image source~pixabay CCO creative commons
The ‘white sand' Or ‘ice’ as many would call it, diamond is one of the most popular allotrope of carbon due to its application in the production of jewelries and in the industries. Diamond has a very lustrous appearance and being able to disperse light, this gives is a very glittering nature and hence a very expensive stone. In the industries, diamond Is used in cutting, drilling and polishing of surfaces. In diamonds, the four electrons available for covalent bond formation in carbon are used up in bond formation to produce an extremely hard non-conducting compound of a stable network of covalent bonds, forming the tetrahedral shaped diamond.
Graphite
Graphene-graphite-benzene chemistry image source~pixabay CCO creative commons
Unlike diamonds, only 3 of the 4 valence electrons of carbon are used in bond formation in graphite, hence graphite can conduct electricity, the electricity is conducted along the plane of the layers, this is because each carbon contributes one electron to a delocalized system of electrons that is also part of the chemical bonding, the delocalized electrons are free to move throughout the plane. Graphite does not have such shiny appearance as diamonds and as such is not used in the production of jewelries, most popularly, it is used in the production of pencils due to its ability to produce dark lines when dragged softly on a paper. Graphite powder is also used in the production of lubricants. It is the most stable allotrope of carbon and is more reactive than diamond because the reactants are able to penetrate between the hexagonal layers of carbon atoms in graphite
Amorphous carbon
These are non-crystalline allotropes of carbon usually containing crystals of graphite-like or diamond-like carbon. During the decomposition of carbon containing compounds, smokes which later settles as soot or carbon black are informally regarded as amorphous carbon.
Graphene—Our own Vibranium?
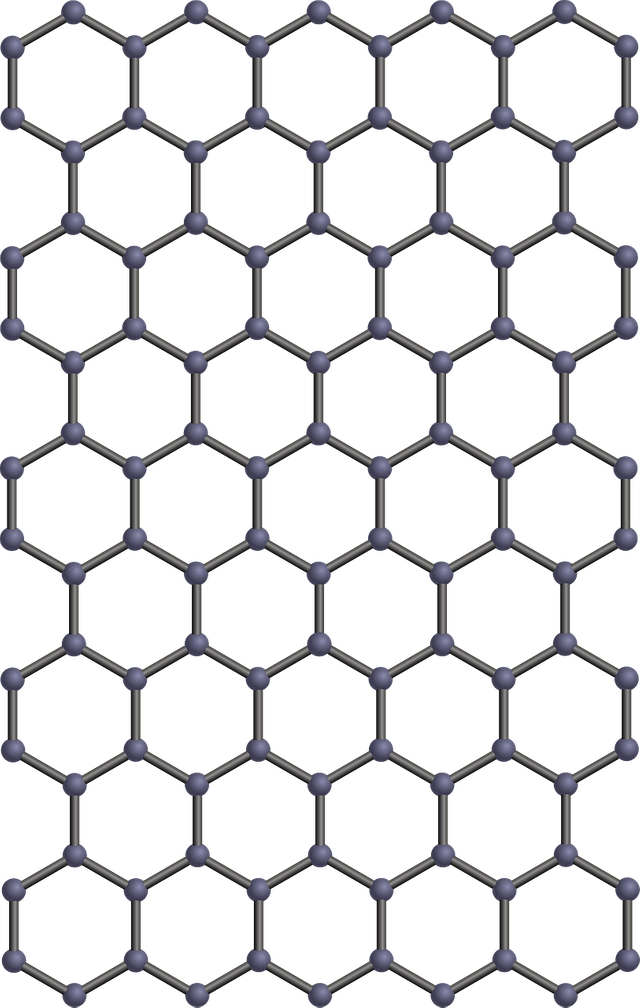
Graphene is made up of a one atom layer of graphite, the carbon atoms in graphene are laid out flat like billiard balls on a table, unlike diamond whose crystalline structure has a three dimensional (3D) crystal lattice, the crystalline structure of graphene is 2-dimensional in structure (2D) with each layer made of hexagonal rings of carbon giving it a honey comb-like appearance.
No more Silicon? Maybe!

Graphene might replace silicones in our electronics technology. wikimedia commons licence ~Creative Commons Attribution-Share Alike 3.0 Unported
Over the past couple of centuries, industrial technology had developed so much and the rate of these developments is only on the increase, one of the most interesting aspect of these developments is flexibility and what I would call
‘miniaturization’. Recent forms of the gadgets used in the past has come to be very much smaller than what they used to be, from the ‘very hard to use’ computers which could completely occupy a room to the very much slim laptops and a much smaller palmtops and smart phones, everything about our electronics technology have been centred on creating high performance in the smallest possible structures , the advent of Silicones shined a huge light on this goal and over the past years and contemporarily, silicon is regarded as the ‘miracle metal' of the electronics world, engineers could achieve much flexibility using the silicon.
However, technology has advanced so much and the thirst for more flexibility has increased over the past few years, a taste which supersedes that presently offered by silicones This has led to several researches on several exotic new materials including germanium, black phosphorous and silicene in an attempt to produce a material with more strength, flexibility and conductivity than the presently used silicon, researches as regard this is continuous but a major set-back being the high cost of the projects amidst very gradual success rate. The advent of graphene has been a big boost to this project and graphene if successfully developed would not only provide an answer to these questions but might bring an end to the current ‘silicon-oriented' semiconductor electronics technology.
Strength and flexibility — just what we wanted!
Being over 200 times more flexible than the currently used silicon, the graphene offers salivating opportunities to the electronics technology industries, this what we always longed for. Graphene is the strongest material ever tested with an intrinsic tensile strength of about 130GPa and a young modulus of 1TPa, this is unlike it’s parent compound —graphite. Graphene provides a strength which surpasses that of diamond, this would puzzle a normal scientist, this is because diamond is actually very much stronger than graphite whose atom makes a graphene, but even though the carbon layers inside a stick of graphite could shave off so easily, the atoms within those layers are very tightly bonded, hence the graphene which is made up by these tightly bonded atoms is overly stronger than graphite and diamond with a strength of about 200 times that of steel.
Due to this flexibility, the graphene can be stretched so much without breaking apart, this is because the flat plans of carbon atom in graphene can flex relatively easily without the atoms breaking apart, this property accompanied by its amazing thinness would enhance the production of smaller gadgets with more flexibility and strength.
The amazing conductivity property of graphenes.
Probably you could remember your tutor telling you to keep your desktop computer in a cold room, or at least provide a good external cooling system to complement the in-built cooling system, eventually, many would have forgotten this era, but fortunately, our present day miniaturized laptops still has this cooling system. Many times you’d touch some parts of your cell phones and feel a very reasonable amount of heat, some would exaggeratedly say that ‘this heat is enough to boil an egg’. But strictly speaking, they are not far from the fact, as when compared to professional heating equipment, the heat produced by our electronics gadgets (which are not built to do so) is enormous and uncalled for.

over-heating of devices by dissipation of heat from the silicon semiconductors may lead to burning of the device image source~pixabay CCO creative commons.
This dissipation of heat can also be seen in electrical wires and in cases of ‘overload’, these wires Maye get burnt and our phones may ‘crash' from over-heating, this situation I’ve experienced so much. The current silicon wires and electronics chips dissipates so much heat which is unbearable, presently, one of the qualities of a good electronics gadget or wires is the ability to handle a reasonable amount of heat without dissipating a noticeable quantity of heat and hence do not produce a noticeable hotness.
The graphene would produce super quality gadgets and wires as regards this, it posses a feature of an impressive heat conductivity and electrical conductivity, it’s heat carrying capacity supersedes that of Silver and copper and can not be compared to diamond or graphite. Heat and electricity has something in common, which is the transport of charges, hence a good conductor of heat would also make a brilliant conductor of electricity, the graphene is not an exception to this. The flat hexagonal lattice of graphene offers little resistance to electrons which moves through it quickly and easily, conducting electricity better than copper, the electrons in graphene can go further without crashing or being interrupted, this interruption being the main cause of electrical resistance and hence dissipation of heat which is the main reason why our phones, wires, computers and other gadgets heats up, this would become a story for history in a graphene-oriented technology system.
Other properties of Graphene.
I guess you would be already satisfied with the already mentioned properties of graphene, but we’re not done yet, the graphene has many other interesting properties apart from flexibility, strength, mobility and awesome conducting abilities, graphene having such thinness with fewer atoms to battle, photons would easily penetrate them, hence the graphenes are likely to at least be translucent if not transparent, this property combined with its conductivity would revolutionize our LCD technology and touch screen technology in our phones and other gadgets which uses a touch screen system.
Moreover, the enhanced movement of charges in graphene provides the possibility of producing even faster microchips, thus graphenes not only provides enhanced electricity conducting opportunity but also simplifies the manipulation of these charges to suit our present day speed-oriented technology, this would be very good news to the artificial intelligence discipline as the machines will be overly faster than humans while they are already smarter than humans.
But don’t jump yet!
With so much already said about graphenes, you’d be excited about the prospects of a graphene transistors and microchips, graphene wires and all that, but unfortunately, these properties have only been tested out on a small scale, amidst gradual success made in the quest to produce graphenes with these properties in a large scale, while even more exciting properties of graphenes are yet to be uncovered there still lies much more work to be done before we can get a fully working graphene technology which may take a space of years and even decades to come by. Other set-backs being the cost effective aspect of this research as it is estimated that a cost of over $2billion would be needed to develop a sufficient graphene technology. But nevertheless, this compound as well as it’s discoverers deserves all the accolades they get.
REFERENCES
1.Graphene~wikipedia
2.Graphene: The next S-curve for semiconductors~mckinsey
3.Grapene~explainthatstuff
4.Applications of graphene
5.Allotropes of carbon ~Wikipedia
If you write STEM (Science, Technology, Engineering, and Mathematics) related posts, consider joining #steemSTEM on steemit chat or discord here. If you are from Nigeria, you may want to include the #stemng tag in your post. You can visit this blog by @stemng for more details. You can also check this blog post by @steemstem here and this guidelines here for help on how to be a member of @steemstem. Please also check this blog post from @steemstem on proper use of images devoid of copyright issues here.

Great post on graphene @joelagbo I love the way you dived into details and still kept it simple -- I have have not watched Black Panther
It's my pleasure, I guess this came late following my promise to write on it, had many posts many post at hand, so I couldn't post it earlier even though I've written it some times ago.
It's not really needful, I just thought of something as mysterious as the graphene and the black Panther's vibranium was the only thing that could pair up, but you got to watch the movie bro.
Thanks for stopping by.
Hi @joelagbo!
Your post was upvoted by utopian.io in cooperation with steemstem - supporting knowledge, innovation and technological advancement on the Steem Blockchain.
Contribute to Open Source with utopian.io
Learn how to contribute on our website and join the new open source economy.
Want to chat? Join the Utopian Community on Discord https://discord.gg/h52nFrV
Maybe with graphene, I can finally achieve one of my wishes to create something similar to Logan's Adamantium (maybe stronger than Vibranium)
Nice piece buddy
The graphene would enable you produce magnificent things you never imagined of, you might be the next super hero of the Tech world with graphenes.. Lol
Thanks for stopping by.