Different states of matter
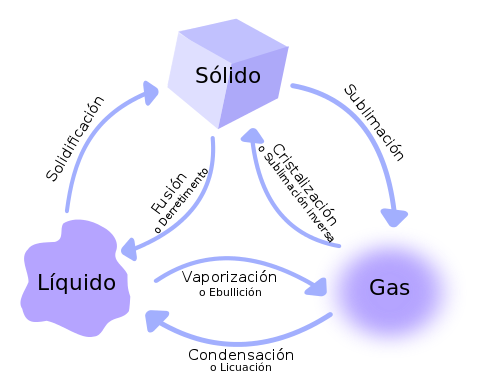
When a body, due to heat or cold, passes from one state to another, we say that it has changed its state. In physics and chemistry, a change of state is defined as the evolution of matter between several states of aggregation without a change in its composition. For example, in the case of water, when it is hot, the ice (water in solid state) melts and if we heat liquid water we see that it evaporates. The rest of the substances can also change state if the conditions in which they are found change. Besides the temperature, also the pressure influences the state in which the substances are. The changes that occur in the matter are fusion, vaporization, crystallization, solidification, sublimation and condensation.
Fusion:
If a solid is heated, there comes a time when it becomes liquid. This process is called fusion. The melting point is the temperature that a solid substance must reach to melt. Every substance has a characteristic fusion point. For example, the melting point of pure water is 0 ° C at normal atmospheric pressure.
Vaporization:
If heat a liquid, it is transformed into gas. This process is called vaporization or evaporation. When the vaporization takes place in the whole mass of liquid, forming steam bubbles inside it, it is called boiling. Also the boiling temperature is characteristic of each substance and is called the boiling point. The boiling point of water is 100 ° C at normal atmospheric pressure.

Crystallization:
The crystallization or reverse sublimation (regressive) is the change of matter from the gaseous state to the solid state directly, that is, without going through the liquid state.

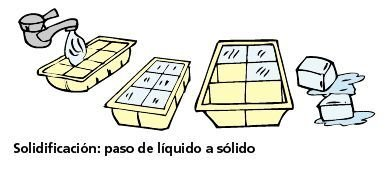
Solidification:
In the solidification the change of state of the matter from liquid to solid occurs, due to a decrease in temperature. This process is the reverse of fusion. The best example of this change is when you put a glass of water in the freezer. When leaving it for a few hours there the water turns into ice (liquid to solid), due to the low temperature.
Sublimation:
The sublimation or volatilization is the process that consists in the change of state of the solid matter to the gaseous state without going through the liquid state. The reverse process is called reverse sublimation; that is, the direct passage from the gaseous state to the solid state. A classic example of the substance capable of sublimation is dry ice.

Condensation:
Condensation is the change of state that occurs in a substance when passing from the gaseous state to the liquid state. The temperature at which this transformation occurs is called the dew point.

You've forgotten Plasma and Bose-Einstein althought both uncommon.