NITROGEN --- A BIT OF CHEMISTRY AND MORE OF LIFE.
Today, my friend reminded me of an incident that happened in one of our chemistry practicals during my college days. On that day, our chemistry teacher was to introduce us to the gas Nitrous oxide. I remember her saying that the nitrous oxide is also called laughing gas due to its ability to make someone laugh uncontrollably.
We doubted her. Unfortunately, she was called out by our principal, leaving us alone in the lab. Hahaha.. The thought of what happens next makes me laugh. Yes, Oscar is his name. You know that boy in every class that claims the tough guy in the class, that's Oscar. Oscar, in a act to display his toughness went and opened the innocent looking container on the shelf across the room. That was when hell broke loose. Like someone who is possessed Oscar fell down and started laughing uncontrollably.
We joined him, without even knowing the reason he was laughing. For 10mins he was still laughing, that was when we knew things have gone out of hands. We had to rush to the principal's office to call our teacher.
Some might say it serves him right, but I see this event as the best introduction to one of the most important element in the universe, and a display of how powerful it is. In this post I will be bringing this element to light and I promise to make this discussion as interesting as possible.
INTRODUCTION
Whenever we talk about the air, what comes to mind is oxygen, and because of that most people think that oxygen makes up a greater percentage of the atmosphere. That's a very wrong assumption. In my research on the Nitrogen I learnt that Nitrogen makes up 78% of the earth's atmosphere. It doesn't end just there, it is also the 7th most abundant element in the solar system. This can be used to run cars too according to The Drivers Checklist
Nitrogen is the most abundant element in the earth's atmosphere
After oxygen, hydrogen and carbon it is the most important element in our body, about 3% of our body is made of nitrogen. It is also a fact that it is one of the major constituent of our DNA and RNA.
Let us get to know about this element. In the next subsection I will be talking "Chemistry", just bear with me, I will try my best to make it interesting.
NITROGEN
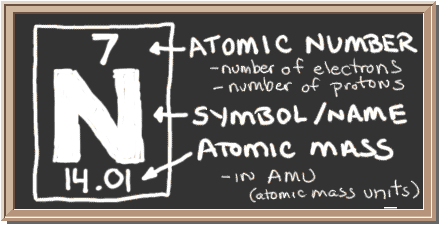
The element nitrogen is symbolized with the letter 'N' and it's has an atomic number of 7 and a mass number of 14. Nitrogen is in group 15 (yes, it's no longer regarded as group VA) in the periodic table. Members of Group 15 are known as pcnitogens, due to the choking effect they cause when inhaled.
Nitrogen has an atomic number of 7 and mass number of 14
Before we get to know about this element, it is worth noting that every element possesses characteristics that is determined by its atomic number. To understand this, let's define an atom.
An atom is the smallest amount of matter that can retain it's identity as a chemical element. An atom is made up of the proton, neutron (which are found in the nucleus of the atom) and electron which orbits about the nucleus. The proton has a positive charge and the number of the proton in an element is equal to its atomic number, it also defines the element. The mass number of an element is given by the sum of the protons and the electrons of the element.
With the help of models like Rutherford model we can place electrons of an atom in their respective shells. Remember that an element is stable when it achieves a duplet or an octet state, i.e when it's outer most shell is completely filled. This is called a noble state. At this state an element doesn't react with other element.
Trying to represent the Nitrogen using the KLMN method, we will observe that the outermost shell has only 5 electrons, thus it still needs 3 electrons for the shell to be completely filled. Therefore it either donates it's 5 electrons to another element for it to be achieved noble state or it receives three electron from other elements to be stable.
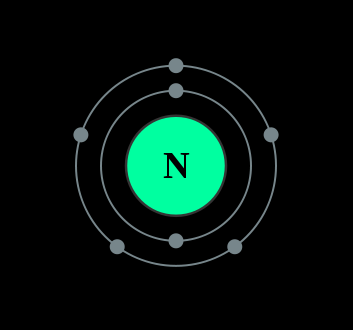
It has two valence numbers +5 and -3
This leads us to what is know as Valence. Remember that all elements want to be like the noble gases that is the reason why they react or bond with other elements so that they might either lose, or gain electron(s) and thus achieve duplet or octet state of the noble gases.
Their readiness to react with other element is dependent on the number of electrons they have. Just think of it, an element that has only one electron in its outer most shell can readily give up it's electron an achieve stability than an electron like Nitrogen that has five electrons in its outer most shell. This readiness to combine with other elements is called "valence".
Nitrogen can give it's 5 electrons or gain 3 electrons to achieve a noble state, thus because of that, it has two valency numbers, +5 and -3. Excluding helium, argon and neon, nitrogen can bond with every other element in the periodic table. Nitrogen cannot exist as an atom thus it exist as a molecule. Apart from being found naturally, nitrogen gas is produced industrially by fractional distillation of liquid air.
AN ESSENCE OF LIFE
As I made mention earlier on, Nitrogen makes up 78% of the atmosphere. It is expected that an element of that quantity should be able to support life. Well, it does, but not the way we think. In 1772, Daniel Rutherford discovered that a part of the air doesn't support combustion, even when he isolated it from air and allow a rat to inhale it the rat choked to death. He observed that the nitrogen gas, which is odorless and colorless displaces oxygen and causes asphyxiation.
You may ask, since it causes someone to choke to death how does it support life? Let me give you my reasons.
You know that our DNA and RNA contains our genes and when man procreate those genes are shared to the offspring. Remember also. that it is in the DNA that information needed for reproduction. growth and development are stored in and they are passed to the offspring during reproduction. What if I tell you that nitrogen is one of the major constituent of our DNA?
Even the protein we eat for growth consist of so much nitrogen. Same with amino acid. Thus, these basic things we need so as to keep existing and be who we should be in this world contains nitrogen. Therefore, it is an essense of life. Plants need them too. Nitrogen is a limiting nutrient, it is observed that unlike some other elements, when nitrogen is added to a plant, it has an effect on the plant growth, when it's not added it's absence is felt.
At this point a question we should ask ourselves is, since animals(man) and plants consume nitrogen so much, shouldn't there be a way those nitrogen are replenished so as to maintain balance in the ecosystem? This question now leads us to Nitrogen cycle.
NITROGEN CYCLE
We will be considering the two nitrogen cycle: The one that occurs on land and the other that occurs in the sea.
TERRESTRIAL NITROGEN CYCLE
Just like most of the things we use, we might not know how to produce them but that doesn't prevent us from consuming them. In that manner, plants and animals use nitrogen but they don't know how to process atmospheric nitrogen to the form that can be processed by their system nor do they know how "fix it" back to the atmosphere.
There are basically three processes involved in the nitrogen cycle, they are ammonification, nitrification and denitrification. These processes are performed by bacteria like Rhizobium trifolium and other single-celled prokaryotes which are found in soil or in the body of plants. The first step is the conversion of the atmospheric nitrogen into ammonia by these bacteria. This ammonia produced is used up by plants to make organic molecules. Animals including man gets the nitrogen when they eat these plants.
After making use of the nitrogen, animals excrets it out as waste. These waste are acted upon by bacteria and the nitrogen in these wastes are converted into ammonia, then to nitrates and nitrites. Finally these nitrates and nitrites are converted back to Nitrogen gas (N2). This process is called nitrogen fixation.
MARINE NITROGEN CYCLE
The processes are performed by marine bacteria and archaea. What happens is that these compounds that contain nitrogen fall to the floor of the ocean in the form of sediment. Over times these sediments are compressed to form sentimental rock.
Tides or storms might lift the sedimentary rock to the land. And as the sedimentary rock wears on earth the nitrogen gas is given off to the atmosphere.
HUMAN ACTIVITIES AND HOW THEY AFFECT THE CYCLING OF NITROGEN
Humans always try to always think of an artificial solution to natural phenomenons. Man has come up with ideas on how to fix nitrogen back into the atmosphere. This artifical nitrogen fixation process is called Haber-Bosch process. In this process H2 reacts with N2 to form ammonia. This ammonia is used in making of fertilizers for plants.
Apart from the use of fertilizer, man also release nitrogen to the air by burning of fossil fuel. What the atmosphere needs is just atmospheric nitrogen gas, but these other compounds that contain nitrogen which are found in the atmosphere posses danger to man as they do result to acid rain(HNO3) and also causes greenhouse effect(caused by the presence of nitrous oxide in the atmosphere).
Also, excess fertilizers can be washed into lakes and oceans by rain. Remember that nitrogen has an effect on the growth of plant even plants inside the sea. This causes an excessive growth on the number of algae on the sea, very soon there will be limited amount of oxygen in the ocean to breath. This problem is referred to as Eutrophication. And this then leads to a chain reaction that leads to the death of so many aquatic animals.
APPLICATIONS OF NITROGEN
If you have followed this discussion from the beginning, you would guess that nitrogen should have a lot of applications, but I will just be listing a few of them.
Normally, after foods are prepared and package, they face the threat of an oxidative damage which causes the food to breakdown thus leading to spoiling of the food. But with the aid of liquid nitrogen (due to its cryogenic nature) and carbon dioxide it can be preserved and prevented from spoiling even up to a year.
In place of argon, nitrogen can be used in producing of incandescent bulbs since they are quite cheaper than argon.
incandescent bulbs made with nitrogen gas
In engineering, due to its low temperature at room temperature it can be used to shrink fittings such that they can be easily inserted. These fittings becomes very tight as they get back to their normal temperature.
Nitriding which is one of the method of case hardening involves heating steel with ammonia to make it hard. The surface of the steel absorbs nitrogen and thus becomes hard.
Nitrogen is also used in building the internal parts of a computer so as to prevent them from over heating when used for a very long period of time.
Nitrogen in the form of nitrous oxide (i.e laughing gas, remember our introduction) is used as anesthetic in medical field.
CONCLUSION
As expected, there are yet so many things to be discussed about the nitrogen, stuffs like the way it reacts with hydrogen to form ammonia, how it forms nine molecular oxides with oxygen, the urine/fishy smell of ammonia and how it form nitrates when it reacts with metal. Don't worry, I will be writing on that very soon. It would much more fun than this.
But there is a safety warning I would like you to keep in mind whenever you are handling nitrogen. I purposely kept it till now so that you won't forget it. The warning is this, Liquid nitrogen is quite dangerous and it can cause cold burns when it comes in contact with your skin. Even though a phenomenon known as Leidenfrost effect can provide some protection if the exposure is for a short time, am sure its not worth risking it.
Thank you for reading, let do this some other time.
Meanwhile, check out this post: Service Stability System (Everything to Know) by steadymechanic to solve your car related problems.
I also recommend a food tour of the united states, check out top restaurants to visit in Butte as well as restaurants to visit in Newport
REFERENCES
Nitrogen wiki
Dna
Ammonia
Five every day use of nitrogen
Nitrogen cycle
Nitrogen fact
Periodic table
Valency
Nitrogen family
Nice opening anecdote.
I wonder if the Joker ever used nitrous oxide on his victims. Sounds like the kind of thing he'd do!
Lol, Yeah @alexander.alexis, Oscar can do a thing like that. I believe it was karma that paid him back.
it is called hysteria, not laughter per se. Nice try but the initial story does not add up.
I felt that by using the intial story I will kind of get the discussion started in an interesting way.
"Hysteria"... I won't forget that. Thank you very much @gentleshaid, I am grateful.
Congratulations bro
Thank you @pangoli..
yea I understand. It is good to start with a bit of chitchat, but it is better if the chitchat can add up.
Thank you. I will always keep that in mind.
Thx a lot, man. It's a funny coincidence, cause I posted a short entry about nitrogen about a day ago... but it was definitely a more targeted one :)
I just checked it out. It's a beautiful article. I love the way you introduced it. I will be checking out your future posts, I bet I will learn a lot from them.
Eat more beans, people! We need to start consuming more foods that have a natural symbiosis with nitrogen-fixing bacteria and reduce our dependence on fertilizers. The current farming model isn't sustainable!
Good write-up!
Exactly, @charitybot. It really amazes me that we prefer to eat foods that are less nutritious than foods that are much nutritious like beans.
Sadly to the large producers it's all about the $$$.