Focus on Chirality.
Two molecules, which reflect each other, only to recognize them separately, special light can be seen to see who is left and who is right-handed.
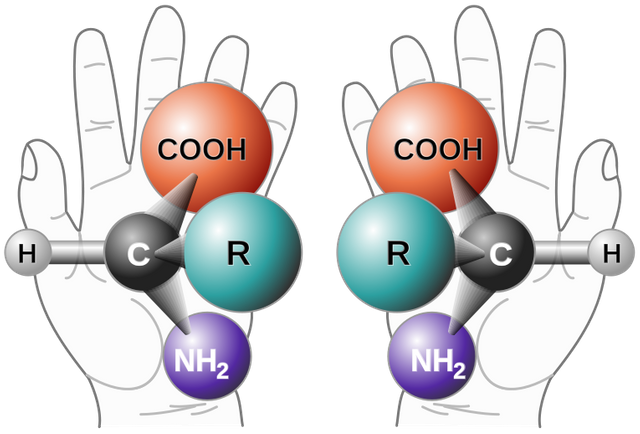
Have you noticed a fun thing? Try to put your right hand glove to the left and see. Not right, is not it? Although the two gloves look different but not the same.
We are facing this strange thing in chemistry. Just as the right arm can not be left on the left hand, but the right arm and the left hand are the mirror of each other, exactly the same way there are many molecules that are not identical even with the reflection of each other. This special quality name is Chirality or handiness in a handful of English. The following figure gives some samples of chivalry.
Figure 1: Although the contrasting Chirality molecules look the same, their formation has a slight
difference. Can those differences be identified in the above pictures? They are a reflection of each
other but not uncommon.
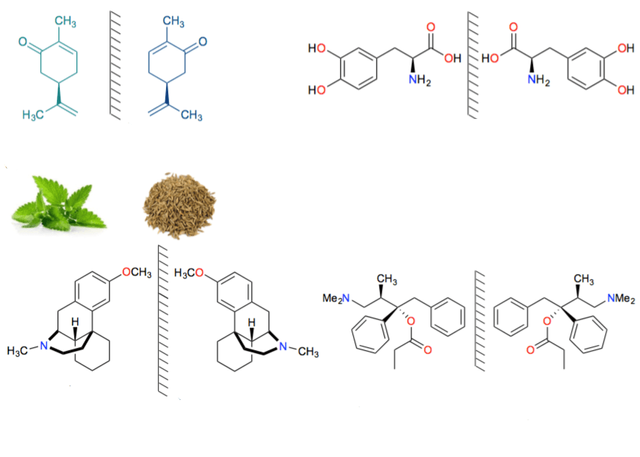
l- karvonen's smell is just like the mint leaves and its reflection is like the d- karvonen's smell jawane. Our nose, however, can differentiate between two molecules. Another example is the predominant dopamine predator l- dopa in the body of neurotransmitters present in our body or two neurons . It is found in some kind of chirality of the body. The opposite of chivalry dDopa but inactive in our body. Again, let's talk about Darwin's painkiller medicine. The contrary chirality of Novarad (Darvon's opposite Novrad) is not painful, but rather it works as a cough medicine. Dextromethrofan is found as a medicine for cough but its image is not available as levomethragon medication. However, it removes pain. Looking at these examples, they can see that they look just like each other, not exactly the same. The relation between those molecules that are called their enantiomer
There are many molecules that are not even indistinguishable from each other's reflections. This special quality name is Chairality
Many drugs are sold as an antivirus because their other antivirus can be harmless or harmful. l -penicillamine Rheumatoid Arthritis is used for the treatment, but d- phenicillamin is very harmful. In the context of this topic I will say a painful history in the last example. Due to arbitrary use of two types of thalidomide antivirus on pregnant mothers in the late 1960s to the early 1960s, a global crisis arises when many children are born with physical distortion. Although d- lithidomide is used as sleeping medicine, l- lolidomide hinders the normal growth of human liver, causing the child to be born with physical distortion.
Figure 2: Selling many medicines as an antivirus can be very important.
source
Understand that it is very important to understand that any antirecrator should take acetic molecule molecules. But how to judge who is right and who is left molecule? We have hands, but the molecules have no hands. Ever watch karbhona or dopa anuyugalake l to d that has been addressed. What is d and l actually? 1
How to recognize Chirality?
You will not leave the left hand or throw it to you to understand the cricket balls. Those who are in the right hand, are right-handed, and those who left, left left. In the same way, in the molecule, which is left to the left and a common means of understanding right-handed is a kind of light on them, how they are using the light. Light is actually electromagnetic wave. One electrode and another magnetic field rush together from the source - we call it light. The electric field and the magnetic field are in the right angle with which the light goes, and also themselves are at right angles with each other. For this discussion we have to keep the matter of electricity only. There is a special religion of light, Which helps us to understand that there is no molecule left or right left This religion is called polarization. On the path of light, its electrode tremble in any direction, it is called light polarization. The problem of understanding whether any molecule left or not to the left is left or not, is not polarized. The general light (such as the light that flows out from the bulb) is not polarized. In other words, one observer, standing in the direction of light, will see that the direction of the light of the light is changing arbitrarily, there is no rule of rules. But if a particular substance called polariser (like most sunglasses) is made in the light of normal light, then we can get pane-polarized light or linearly polarized light. Because, This polarizer allows the electrode to move only through one floor (indicating this floor along the direction of the electric field and the direction of the motion of light). The polarizer stuck to the part of the normal light wave, which was flowing out of this fixed surface.
Who is left-handed and right-handed anudera understanding of the polarized light goes out a way to use them to meet
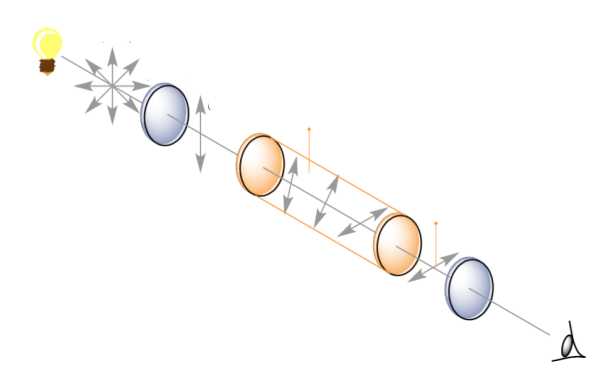
Now, if polarized light can be sent through d-glucose solution, then the polarized light turns to the floor of the polarization (Figure 3). d-glucose is therefore called dextrorotatory (d). Just like l-glucose levorotatory (l). The following figure shows the subject. For the convenience, we will call the Dexterotator in the right arm and the liverotator as left arm.
Figure 3: Polarization of the surface turns into the presence of chiral molecules.
It is so difficult to understand why d or l is named chairlal molecules. But the plane of Polarized-Polarized Lighting turns around like this? What is there in these molecules? The answer is not easy at all. Let's try to answer this, but before that let's try to understand the polarization of light more.
Two more stories about the polarization of light
Polarization is a special religion of lightning. How can the plane-polarized light be found, I have already seen. For now, just forget about the magnetic field, just like the electric field. In fact, when the light vector of light vibrates on the same plane, it is called a plane-polarized light [Figure 4 (a)]. It is also given to the viewers who will look at the direction of light [Figure 4 (b)].
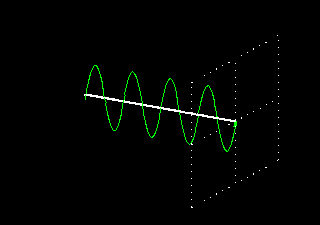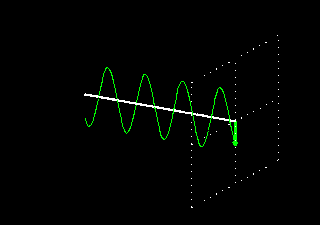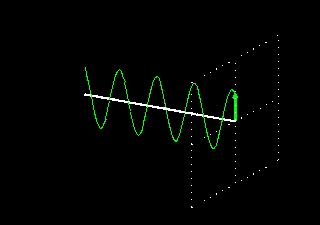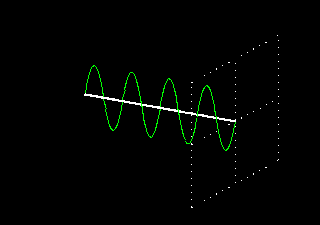
Figure 4: (a) Vertical vectors of plane-polarized light are fluctuating. The magnetic field
fluctuates in its vertical direction but it is not shown here.
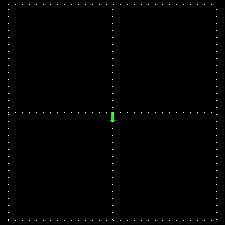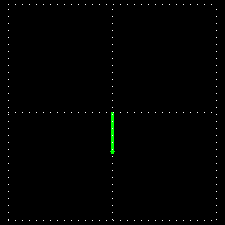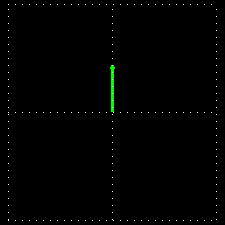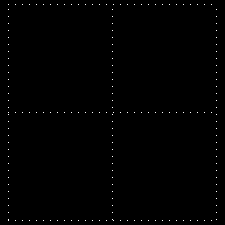
Figure 4: (b) As you stand on the direction of the illumination, it will show
And in some cases, polarization is done when light bulbs go round, for example, see Figure 5. The circularly polarized light of this circular characteristic is that they are inherently the Cyril Looking at the direction of the light, in view of the viewer, Figure 5 (a) right-handed light and Figure 5 (b) left light. In fact, any such screwdriver structure (helix) is inherently chairlal.
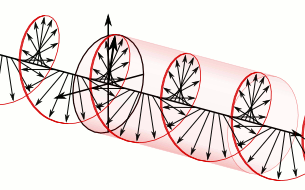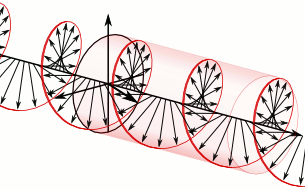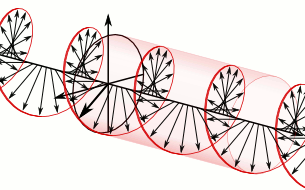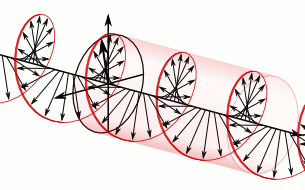
Figure 5: Circularly polarized light is inherently chairlal. The radius vectors are shown with arrows.
(A) Right arm circularly polarized light
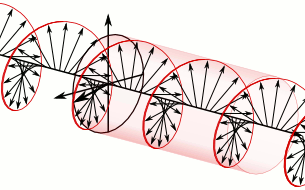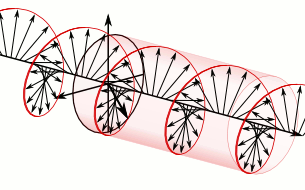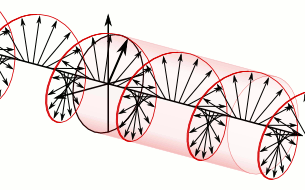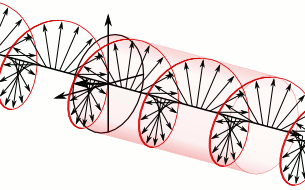
(B) Left arm circularly polarized light. To understand how someone will look at the path of light,
notice where it is crossed with two arrows. In the case of right-handed light, black thick arrow is
rotating right and in the case of left-handed light it is turning left.
Any plane-polarized light wave can be seen as the sum of two equal amplitude and phase circularly polarized light vector, a left circularly polarized light and the other right-circularly polarized light. It will be clear if you see it in the film.
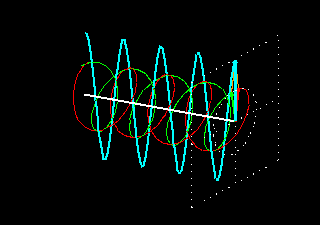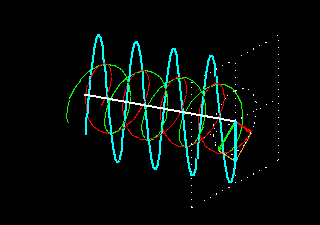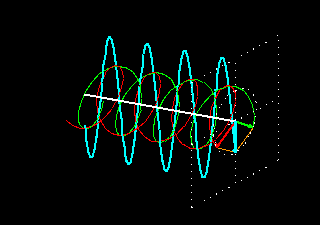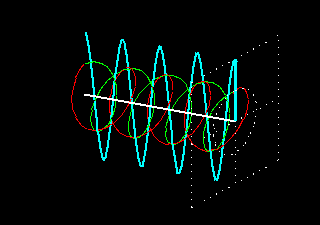
Figure 6: (a) The blue velocity of the plane-polarized light is visible in the vector. These vectors can be broken into two circularly polarized light waves, green vector circularly polarized light vectors and red-colored right-ventricular circularly polarized light vectors.
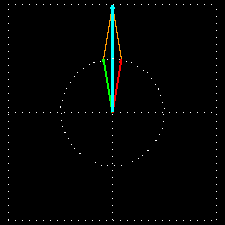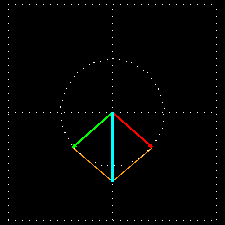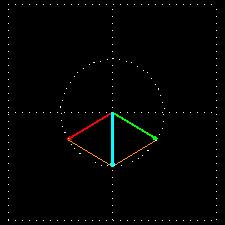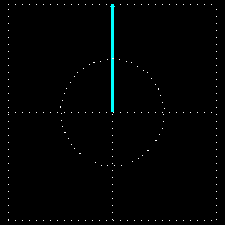
Figure . 6: (b) As it was, in the direction of the light level, the three vectors will be shown how it
feels.
In Figure 6 (a) and (b), you see how the two amplitude right arm and left ventral vector sum of circularly polarized waves form a plane-polarized wave. But suddenly I divided the planell-polarized light into two vectors, why? You will know the reason right away. What happens when this plane-polarized light sends through the solution of l- lysine?
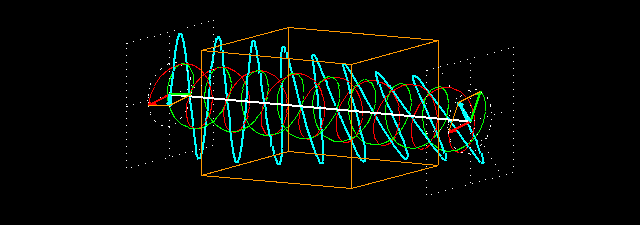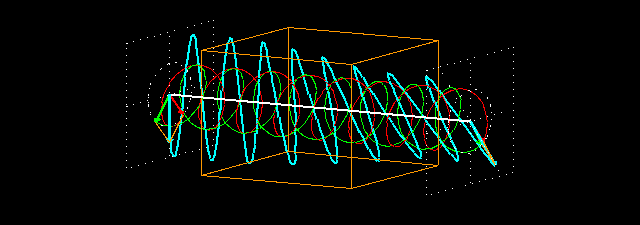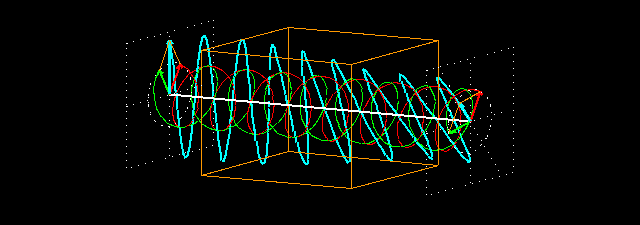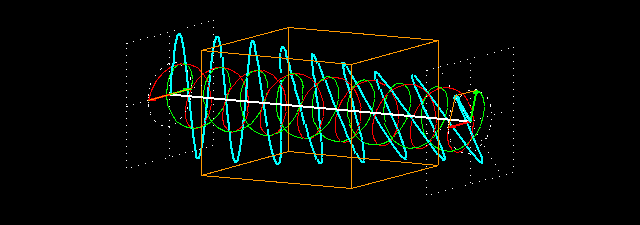
Figure 7: (a) Due to the penetration of l-lucin solution, the right-handed circularly changes the
polarized light wave.
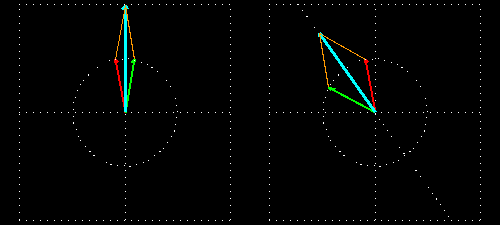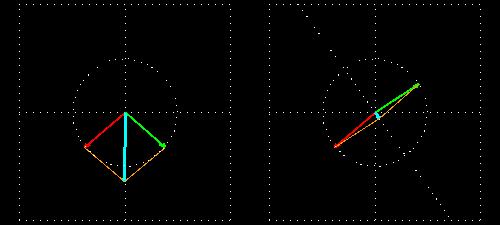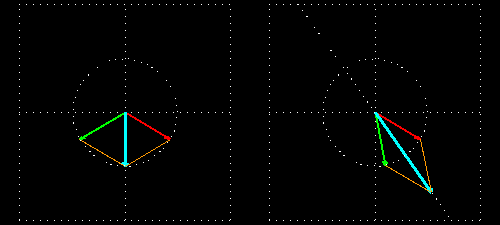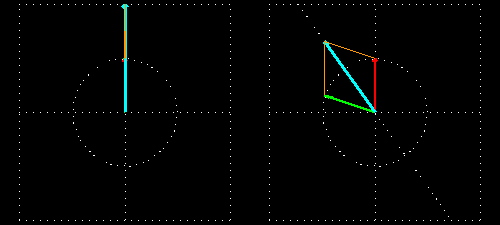
Figure 7: (b) How to show a cross section on the path of light is shown before and after entering
the solution. Notice that the blue-colored plane-polarized light turned to the left.
By sending the plane-polarized light color described above through this solution, the left and right arm of the circularly polarized wave becomes the phase difference, and hence their vector sum and the previous plane-polarized light is not one with the floor, but rather slowly move. In the picture [Figure 7 (a) and (b)] shown how the blue plane-polarized light (which is the sum of red and green circularly polarized light), before going into the solution, is subjected to the rotation of time,
Characteristics of circularly polarized light are those that are easily chairal.
Why the right arm changed the state of light?
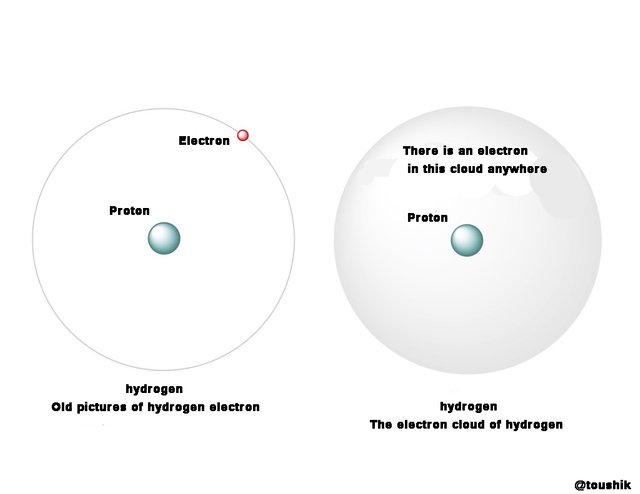
Right-handed circularly polarized light waves, why the phase changed? We must take refuge in understanding the answers of the quantum mechanics in depth. It could be a rough idea even though not inside it. But before that electron cloud and density of electron need an idea. At first I thought about the hydrogen atom as a simple example. Generally, hydrogen is shown with Figure 8 (a). Like the rotation of the sun around the sun, the electron is moving around the nucleus. This picture is not correct but. It is impossible to say that the electron is in a particular place in this way. We can say that we have the possibility of getting electron around the nucleus. This possibility is described as an electron cloud. There is one in this cloud that has the electron. Figure 8 (b) That is giving an idea of the electron cloud.
Figure 8: (a) The old pictures of hydrogen that surround the electron proton. (B) According to
Quantum Mechanics, it is impossible to show hydrogen electrons in a particular place in a
particular place, but instead it can be found around the proton. The area of this possibility can be
described as an electron cloud.
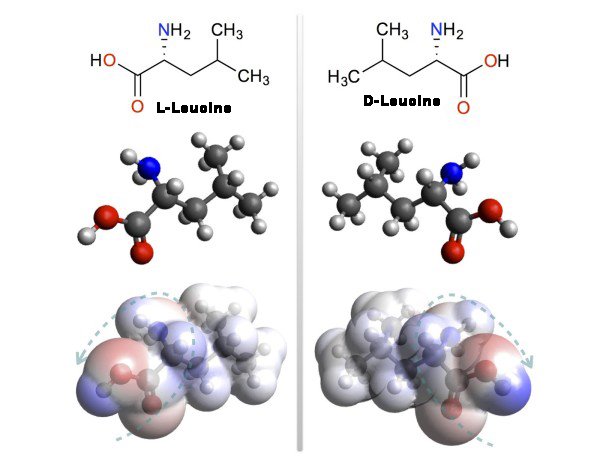
Thinking about exactly the same kind of Chiral amino acid molecule Leucine. All carbon atoms, oxygen, hydrogen, and all electrons around nitrogen atoms have its own electron cloud. The electron cloud is the most likely to have an electron and where less is known with electron density. Figure 9 shows the possibility of having electrons around Leucine where there is less red and where the probability is less blue is shown.
Figure 9: The structure of l- and d-Leucine - (a) L-Leucine composition, ball-stick model and photo
of electron density (b) D-lucein chemical composition, ball-stick model and electron density
picture.(A) and (b) were conceived by carbon, oxygen, hydrogen, nitrogen atoms, respectively,
with black, red, chiaranga and blue balls respectively. At the bottom, the electron density of the same
molecule is shown. Where electron density is more red and where there is less blue color
Following the low electron density of this, the cut-cut green arrow is used to describe the
difference between the two enantiomers in their chirality format.
You can understand that l- Leucine and d- Leucine are the two endocrinates of each other and the appearance of their electron density is also known by each other's Antioxer. Following the low electron density, the cut-cut green arrow is drawn to two enantiomers. Drawing such an arrow is to convey the difference between two enantiomers' electron density chivalry. These arrows are screwed like screws. Here again the discussion of right-handed and left-handed will come. Because, if you look at a screw patch, you will see someone moving towards clockwise (clockwise) - they are right-handed. No other screw moves against clockwise (anti-clockwise) - they are left-handed. In the picture l lcci, this arrow is seen as a left-handed screw and dIt's the right-hand side of Leucine.
Now imagine what happens when the plane-polarized light waves fall on the l- Leucine molecule. As already mentioned, in plane-polarized lighting, we can think that the value of the right and the right hand and the left hand and the left hand light. The electrons of Leucine will tremble in the light of the vibrating light of the light. As a result, the electric electrons that start shaking will create new electrons in the light along with the electric field of light. Magnetic field also created but it was left to the discussion for the time being.
Left arm circularly polarized light waves will easily move the electrons in a screwed road like a left-handed screw (along the road shown with those green arrows). The difficulty will be to the right-hand part of the light. He wants the electrons on the mole to dance on the street like the right-handed screw - but the road will be difficult for him to be left-handed . Then, for a while, the plane will move from the polarized part to the left-handed polarized part of the polarized light, ie, their phase will change. This situation can be thought of with a simple analogy. Think you came to a garden arranged. There is a lot of apples lying around. I want to pick up the apples. You are given two baskets on the condition that the right hand will be collected in one basket and left in another basket. Hold it lYou can do the same as left of Leucine, so you can work fast. After picking up Apple for some time you will see more apples than your left hand in the left hand basket. You can say a lot like this.
What if these two circularly polarized light vector sum? The planar-polarized light wave that comes from this vector sum but its surface is not as old as it is, it turns slightly left to the left. Figure 7 (b) shows that the blue vector is moved to the left due to the green light behind. The opposite of d- lucin will be the opposite, ie the right-hand light will proceed further than left arm. As a result, the plane-polarized light will turn right.
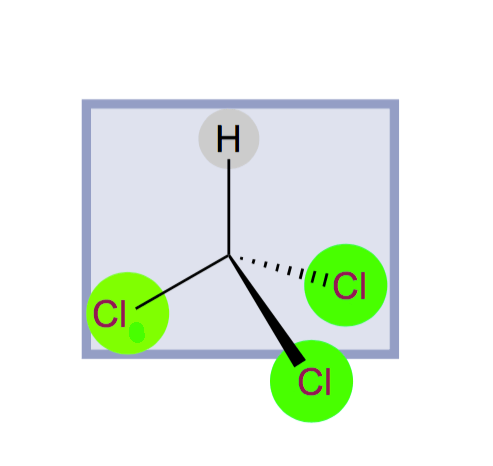
Figure 10: If chloroform molecules conceive the hydrogen and chlorine (1) atoms on a plane, then
the chlorine at 2 and 3 will remain atom over and above the mirror. The mirror in front of this
mirror and the two sides of the chloroform molecules look exactly the same. Such a mirror can not
be imagined in the lysine.
Think of it the same way, d - and what is the proportional mix of l- lysine. For 50 percent of the molecules, left arm circularly polarized light will go ahead and 50 percent of the cases will move right hand. This will eventually result in no vector of plane-polarized light by adding vectors. In the same thought, if I think of a solution whose mirror can be conceived through the center of the molecules (such as chloroform molecules, figure 10), then no rotation of light can be observed.
Since the beginning of the nineteenth century, the mysteries of the Cairali, unveiled by Lewy Pasteur in the middle of that century, Arago, Bio, Herschelle, Frynell, and then in the middle of that century. At the end of the nineteenth century, Acharya Jagadish Chandra himself thought that how the polarized light roams in the presence of the cylindrical molecules. In one of his own papers, Preston quotes that I quote here to give a glimpse of his thoughts.
" It was not a common problem for Farad (the ability to rotate the polarized light of some solutions), and I do not know if any explanation has ever been given. It may also be possible that the soluble polarization becomes polarized in the form of a special structure of molecules when the molecules move freely through the process of lightening, just as the polarization of liquid matter is the result of the determination of the stabilization. "
It is quite possible to understand that his thoughts were almost correct.
Reference
Absolutely fantastic post. I love chemistry; I have a masters in Chemical Engineering.
I also love symmetry in Mathematics, Physics, Chemistry and basically everything else. When I first took Organic chemistry, Chirality was a concept that drew me in like a moth to a flame in a similar way to abelianization in mathematics. Your article here covers everything that anyone would never care to know about Chirality without getting to technical which is extremely admirable.
I like the whole science world.
Thanks for your comment.