How to Earn a Nobel Prize Part 3: Greg Winter
To celebrate SteemSTEM’s launch of https://steemstem.io, I’ve been writing a series of posts on the recently announced 2018 Nobel Prize in Chemistry. This year’s prize was awarded to Frances H. Arnold for pioneering the first directed evolution of enzymes, and to George P. Smith and Sir Gregory P. Winter for developing phage display 1. The award was a major story in a bunch of news outlets, but I found most of the pieces were scant on details. They only gave a cursory overview of the techniques that the scientists spent a major portion of their lives developing. Fortunately, @SteemSTEM has given me the platform to remedy that problem. Each entry in this series will focus on one of the scientists’ contributions, try to explain that contribution, and show why the scientist deserves this prize. Today we’ve gotten to the last of the bunch: Greg Winter and humanizing antibodies.
Who is He?
All you European Steemit users can rejoice because we’ve finally gotten to the non-American recipient :P. Greg Winter was born and educated entirely in England [1](https://en.wikipedia.org/wiki/Greg_Winter). He got his PhD from the MRC Laboratory of Molecular Biology (probably the best molecular biology institute in the country) and pretty much stayed there for the rest of his academic career. Unlike with George Smith, he didn’t develop his Nobel Prize winning work during a [Magical Sabbatical](https://steemit.com/steemstem/@tking77798/how-to-earn-a-nobel-prize-part-2-george-p-smith-and-phage-display). Instead, his research gradually focused on finding a way to humanize antibodies for therapeutic purposes and really took off once he incorporated phage display. He is currently a Professor Emeritus (read: semi-retired) at the MRC Laboratory of Molecular Biology in Cambridge with no major publications since 2012. He’s only 67 (which is like 40 in scientist years) so I doubt his low publication record is indicative of him winding down. In fact, he has spun his research into a number of companies, most recently [Bicycle Therpeutics](https://www.bicycletherapeutics.com/), and is a high ranking member in a number of scientific organizations. Most likely, he is quite active in the biotech industry, but we just don’t have a strong record of it because work in the industry sector gets far less exposure.What did he do?
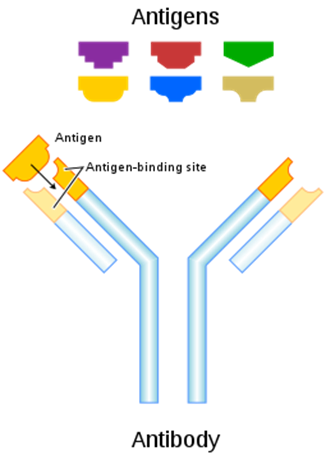
An antibody is a Y-shaped protein that binds to a specific antigen. The ends of the protein determine specificity for a specific antigen (2).
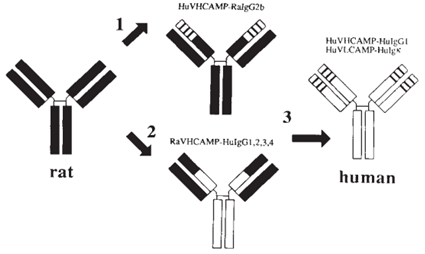
His strategy was to take the crucial portions of the rat antibody that could bind to the protein of interest and transplant them onto a human antibody. It proved to be effective (effective enough for a Nature publication at least :o), but was pretty labor intensive. Had I not already looked into the other Nobel Laureats, I might have just dove headfirst into this paper and discussed its nuances. However, a brief read showed me that this paper just didn’t build upon the work of Frances H. Arnold or George P. Smith. It turned out that I was going to have to do some investigative work to figure out where Greg Winter’s ambition collided with Dr. Arnold and Dr. Smith’s work.
After combing through Greg Winter’s publication history, I found that it took another several years until he officially leveraged phage display to humanize antibodies. In 1991 he published two papers that started using phages to make parts of antibodies and begin showing how they could assemble (4, 5). However, it wasn’t until his 1994 publication, Making Antibodies by Phage Display Technology, that he definitively lays out how his work begins to tie in with that of the other 2018 Nobel Prize recipients 6. In it, he shows how phage display and directed evolution can be used to create an antibody with high affinity for a specific target while not triggering the body’s immune system. What was fascinating to me is that he actually tries to mimic what the body does naturally to generate antibodies. The human immune system combines a series of hypermutatable genes known as V-genes in various combinations to create a bunch of different antibodies and those that bind strongest to the foreign agent are selected. Dr. Winter had phage display do essentially the same thing outside of the body with a collection of V-genes inserted into a bacteriophage.
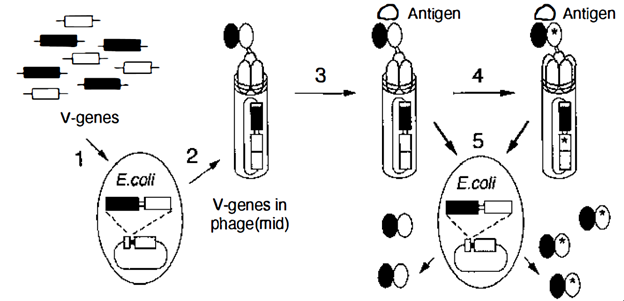
Workflow for using phage display to generate antibodies from a collection of v-genes and selecting those that bind an antigen the strongest 6
This guy’s lab was a publication powerhouse with 10-20 papers a year in the 90’s, so tracking his progress required some skimming of papers with the help of google scholar 7. From what I can tell, the 1994 paper was followed by several years’ worth of scientific reports that fill in the specifics of using phage display to generate antibodies. From proof of concepts showing phage display creating antibodies against specific antigens to focused studies on single aspects of antibody creation he covered a lot of areas. In the 2000’s, his work shifted towards making antibodies more stable with some publications focused on improving antibody diversity. Many of these papers were well received with hundreds of citations, but none achieved the level of importance that his first few papers on this topic did which have thousands of citations each.
Why does this deserve the Nobel Prize?
This work is important because it gave a useful application for the directed evolution and phage display technologies that Dr. Arnold and Dr. Smith developed. To emphasize how big a deal this is, I’m just going to drop this table compiled in the 2013 paper * Drugs derived from phage display * 8
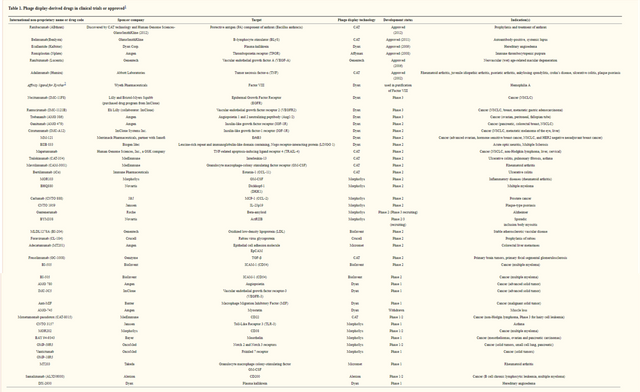
This list was compiled in 2013 and a large portion if it is due to Dr. Winter. Enough said.
Thanks for reading
Well this is the end of the 2018 Nobel Prize in Chemistry series. It was fun to write and I might try another series in the future as it gives me a strong focus for these articles. This one included a healthy mix of both science history and cool science so I really enjoyed it. My only regret is that it took a bit longer than expected. If you have any suggestions for future series, let me know in the comments.
References
(1) https://en.wikipedia.org/wiki/Greg_Winter
(2) https://en.wikipedia.org/wiki/Antibody
(3) https://www.nature.com/articles/332323a0
(4) https://www.nature.com/articles/352624a
(5) https://academic.oup.com/nar/article/19/15/4133/1115775
(6) https://www.annualreviews.org/doi/abs/10.1146/annurev.iy.12.040194.002245
(7) https://scholar.google.com/citations?hl=en&user=QQOy6PIAAAAJ&view_op=list_works&sortby=pubdate
(8) https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3929457/)
Images
All images were taken either directly from the publication referenced or labeled for reuse on Google Images. If any image owner has an issue with this article, please contact me and I will address the issue.


Let me start by saying I'm not a chemist or a trained scientists, so it is a credit to your writing that I was able to actually make associations as I read your piece. When you were describing phage displays and adapting antibodies (humanizing them), I wondered how this work might relate to the challenge of treating autoimmune diseases. Then I checked the list of drugs derived from the phage displays, and saw Belimumab included. This, of course, was the drug introduced with great fanfare some years ago for the treatment of SLE.
Thanks for the introduction to Winter and to his research. I'll definitely be doing more reading about this.
Glad you liked it and thanks for reading!
Ah, it seems I missed the previous installment.
This one is very well written and I particularly enjoyed the humor!
Glad you caught it before it dropped further and further into steemit obscurity :)
Inspiring. I'm still confused regarding the implication of his work though.
It's harder to explain with this guy. He didn't make one single discovery. Rather, he pushed the field forward more than anyone and has directly or indirectly contributed to a bunch of life-saving medications.
Happy to say I remember some stuff from the former articles of the series. :)
So in short, this guy was the first to come up with a practical application of the work of the two other laureates? Am I correct?
Yeah that's it in a nutshell. Others have contributed to this goal, but he devoted his career to this. To my surprise, there wasn't any one discovery that cemented his legacy. It was a series of excellent research and publication over many years that earned him the prize.
Wow! Fully a one-person work? This is rare, especially today where one often relies on earlier works in one way or the other :)
This post has been voted on by the SteemSTEM curation team and voting trail in collaboration with @utopian-io and @curie.
If you appreciate the work we are doing then consider voting all three projects for witness by selecting stem.witness, utopian-io and curie!
For additional information please join us on the SteemSTEM discord and to get to know the rest of the community!
Thanks for the Upvote! Life got in the way of my regular posts, but I'm back on track now.
Hi @tking77798!
Your post was upvoted by Utopian.io in cooperation with @steemstem - supporting knowledge, innovation and technological advancement on the Steem Blockchain.
Contribute to Open Source with utopian.io
Learn how to contribute on our website and join the new open source economy.
Want to chat? Join the Utopian Community on Discord https://discord.gg/h52nFrV
Always appreciate the support!
This story was recommended by Steeve to its users and upvoted by one or more of them.
Check @steeveapp to learn more about Steeve, an AI-powered Steem interface.
Thanks Steeve. I know you're just a machine, but machines need appreciation too.