PhD Candidate Photographs Single Atom, Wins Award
Have you ever wondered what an atom looks like? Imagine if you could shrink down to the size of an atom, what would you see? Turns out it is not as simple as a couple of balls orbiting a central ball like what common atomic illustrations suggest.
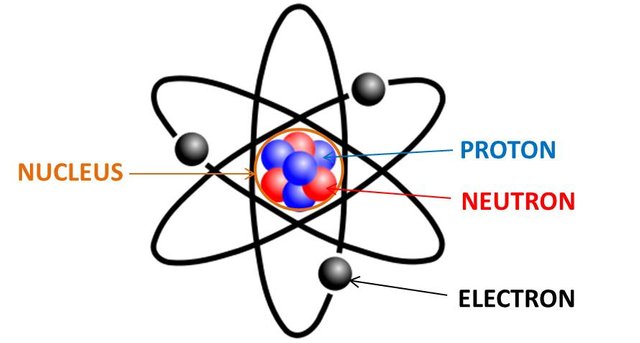
If anything such an illustration could possibly be used to describe the Bohr model of the atom, but is still not an accurate representation of what an atom would look like. The fact is, the electrons orbiting the atom behave more like waves which causes a smearing of the electrons over space. Using the Schrödinger equation with the appropriate potential, one could calculate the probability of finding the electrons in any given location. For the hydrogen atom, it looks something like this for different energies:
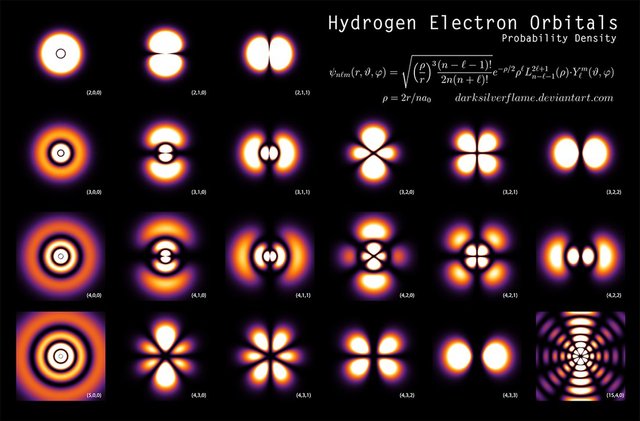
These are the true exotic flowering shapes of an atom. This smearing of electrons is called electron probability density and is of fundamental importance with regards to the physics and chemistry that is observed in our natural world. When scientists performed scattering experiments that was exactly what was observed! Below is an image of a hydrogen atom using something called photoionization spectroscopy.
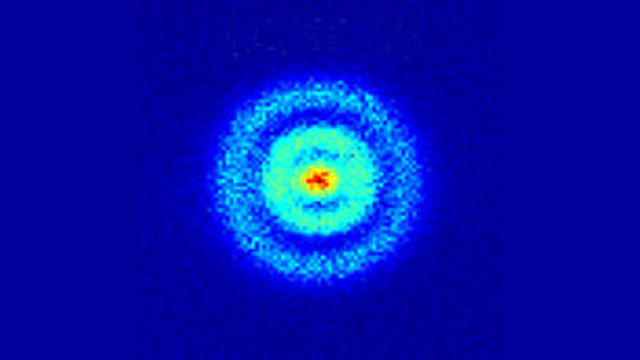
So this is what a man-made machine was capable of visualizing, but what would an atom look like if directly observed by the human eye? Is it possible to directly see an atom using only the physics that the biology of the eye is capable of recognizing, visible electromagnetic radiation (light)? Well, it just so happens that a Ph.D. student at the University of Oxford was able to accomplish this feat. Here is that picture:
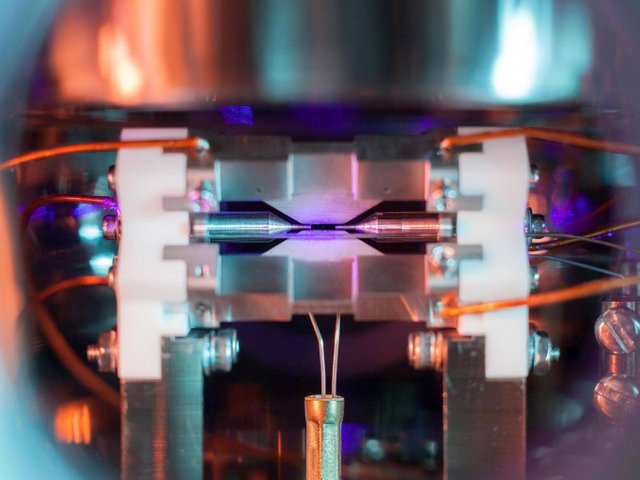
Can you see it? Perhaps zooming in will help…
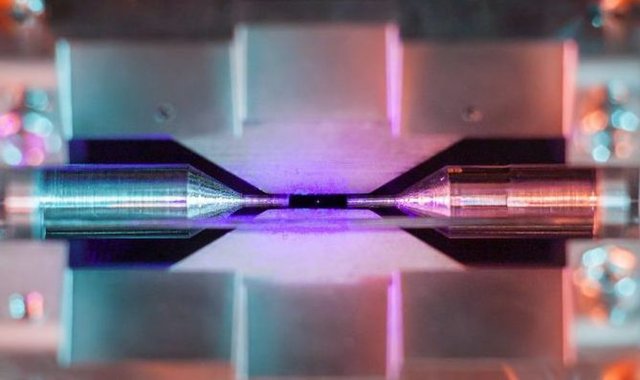
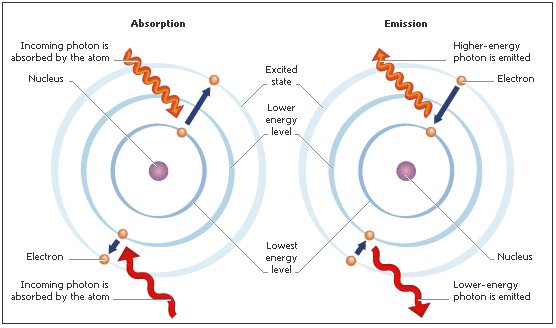
That tiny dot suspended in the middle is a single atom of strontium. The atom appears to defy gravity as it is positively charged and an electric field is holding it in place. The student shined light that is of wavelength within the absorption spectrum of strontium. The atom absorbs that purple light and the electrons enter an excited state, only to relax again emitting light of a longer wavelength and thus the observed color closer to blue.
For a single atom, the light is too minuscule to see. So how did the student do it? By a prolonged exposure to allow the camera to gather as much light as possible making the strontium atom visible in the photograph. The photo won the student an award for science photography at the Engineering and Physical Sciences Research Council. When the student was asked about his photograph he reportedly stated, “[…] when I set off to the lab with camera and tripods one quiet Sunday afternoon, I was rewarded with this particular picture of a small, pale blue dot.” [1] The student is parodying part of a lecture given by Carl Sagan when he referenced this photo of the Earth taken by Voyager 1 from beyond Jupiter as a “pale blue dot”.

It is clear the student is passionate about science and that is reflected in their photograph of strontium making it appreciable by everyone.
Let me know what you think about the student’s photograph below. Stay tuned for more interesting new advances in science and medicine!
Sources are linked within text.
The first photo (looking at other versions of it) is copyrighted. Remember when using images that you need to use the license produced from the original image, not derivative works. As for the other ones, while I ignored the ones looking like they were produced by NASA some of them appear to be copyrighted works as well.
Thank you for looking out to protect the platform and myself. The image you are referring to is actually labeled for reuse and for reuse with modification. For this particular image, it is available on this page on Wikipedia and specifically states permission to use the image. Regardless, I am thankful for your post and will continue to be careful.