Thermochemistry, chemical kinetics, chemical balance and acid-base reactions. Basic concepts.
Hi Steemians!
Energy is very important in all aspects of our daily life. For example, the food we eat supplies energy to sustain life with all its activities and concerns. The relatively cheap availability of energy is an important factor in our technological society. It should be understood that the storage and use of energy on a scientific basis to learn to decrease our dependence on oil and natural gas as our main sources of energy. From this, several concepts are presented, which are explained below:
Thermochemistry
It consists of the study of the transformations undergone by the calorific energy in chemical reactions, emerging as an application of thermodynamics to chemistry. Frequently we can consider that the chemical reactions take place under constant pressure (open atmosphere, that is, P = 1 atm), or at a constant volume (that of the receptacle where they are being made).

Source
- Bond Energy
During a chemical reaction the reagents are fragmented and reorganized. This implies the breaking of chemical bonds and the formation of new bonds. If the total energy of the final bonds is lower than that of the initial bonds, the reaction releases energy into the medium. Otherwise, you must absorb energy from the medium for the reaction to occur.

Source
- Gibbs Energy
The free energy or free enthalpy of Gibbs is used in chemistry to explain whether a reaction will happen spontaneously or not. To calculate the free energy of Gibbs can be based on: the increase or decrease in entropy associated with the reaction, and the sum of heat required or released by it. This energy is represented by the capital letter G. The pioneer of the energy of Gibbs was the American physicist Josiah Willard Gibbs who contributed with the theoretical foundation of thermodynamics.
The procedures that have associated chemical reactions, have some important measures since it is investigated whether a reaction occurs spontaneously or if some interaction with the surroundings is required to occur and is called non-spontaneous. A reaction is associated with enthalpy and entropy at the time of occurrence.
Chemical kinetics
This field studies the speed of reaction of chemical processes depending on the concentration of the species that react, the reaction products, the catalysts and inhibitors, the different solvent media, temperature, and all other variables which can affect the speed of a reaction.
When some substances react they do it slowly, for example iron in the presence of air; others react quickly, such as sodium, also in the presence of air; and there are substances such as paper in the presence of air that would never react without the help of fire, but once the reaction starts it develops quickly.
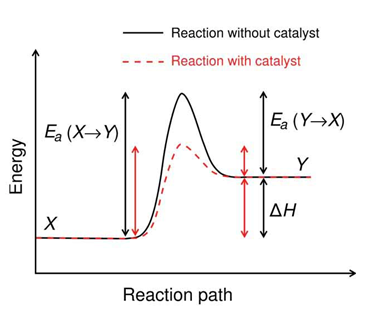
Source
Chemical Kinetics is the branch of chemistry that quantitatively studies the speed of reaction. It also studies the change in the composition of energy states with respect to time. A reaction can be spontaneous according to the thermodynamic laws, but to know if it happens or it does not happen, this must happen in a reasonable period of time. In this case it is essential to note the difference between spontaneity and speed.
- Reaction mechanism
The fundamental objective of the reaction kinetics is the investigation of the mechanism by which a certain reaction takes place. All kinetic problems have a double aspect. In the first place, it is a matter of studying the reaction from an experimental point of view to find the kinetic equation to which the velocity responds. This equation gives us the chemical species whose concentration influences speed. Secondly, it is about proposing a mechanism (that is, a system of differential equations) whose solution leads us to the experimentally found kinetic equation. Then we say that the proposed mechanism is compatible with the experimental data.
- Theory of collisions
The theory of collisions proposed by Max Trautz and William Lewis in 1916 and 1918 qualitatively explains how chemical reactions occur and why reaction rates differ for different reactions.
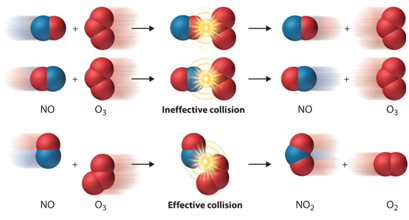
Source
This theory is based on the idea that reactive particles must collide for a reaction to occur, but only a certain fraction of the total collisions have the energy to connect effectively and cause transformations of the reactants into products. This is because only a portion of the molecules have sufficient energy and adequate orientation (or angle) at the moment of impact to break any existing link and form new ones.
Chemical balance
It is a reaction that never comes to completion, because it occurs simultaneously in both directions (the reactants form products, and in turn, they form reagents again). That is, it is a dynamic equilibrium.
When the concentrations of each one of the substances that intervene (reactants or products) are stabilized, that is to say, they are spent at the same speed that they form, the CHEMICAL EQUILIBRIUM is reached.
- Balance constant
The equilibrium constant (K) is expressed as the ratio between the molar concentrations (mol / l) of reactants and products. Its value in a chemical reaction depends on the temperature, so it must always be specified. The expression of a generic reaction is:
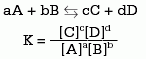
Source
In the numerator the product of the concentrations of the products is written and in the denominator the one of the reactants. Each term of the equation is raised to a power whose value is that of the stoichiometric coefficient in the adjusted equation.
- The principle of Le Chatelier
The Principle of Le Chatelier states that, if a system in equilibrium is subjected to a change of conditions, it will move to a new position in order to counteract the effect that disturbed it and recover the state of equilibrium.
As we have seen, chemical equilibrium represents a balance between direct and inverse reactions. Variations in the experimental conditions can alter this balance and shift the equilibrium position, causing a greater or lesser amount of the desired product to be formed.
The variation of one or more of the following factors can alter the equilibrium condition:
• Temperature
• The pressure
• The volume
• The concentration of reactants or products
Acid-base reactions
Acids and bases are substances that man knows and uses since ancient times. In the eighteenth century it was known that acids had a sour taste in aqueous solution, which reddened the litmus paper and reacted with metals. As for the bases, its lye flavor was known, its ability to turn bluish litmus paper reddened by acids and its neutralizing power to acids. Acid substances react with those of a basic nature, being called acid-base reactions.
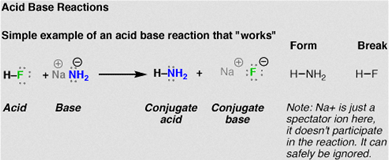
Source
- Lewis's theory
The American chemist Lewis gave a definition about the behavior of acids and bases. According to this, a base would be a species that can donate a pair of electrons, and an acid that can accept them.
The acid must have its electron octet incomplete and the base must have some solitary electron pair. Ammonia is a typical Lewis base and boron trifluoride is a typical Lewis acid. The reaction of an acid with a Lewis base results in an addition compound. Lewis acids such as aluminum trichloride, boron trifluoride, stannic chloride, zinc chloride and iron (III) chloride are very important catalysts of certain organic reactions.
- Neutralization reaction
A neutralization reaction is one in which an acid (or an acid oxide) reacts with a base (or basic oxide). In the reaction a salt is formed and in most cases water is formed. The only case in which water is not formed is in the combination of an oxide of a non-metal with an oxide of a metal.

Source
Greetings! Thanks for your attention.
For more information visit the following links:
- https://chem.libretexts.org/Core/Physical_and_Theoretical_Chemistry/Thermodynamics/Thermochemistry/Thermochemistry_-_A_Review
- https://en.wikipedia.org/wiki/Bond_energy
- http://chemed.chem.purdue.edu/genchem/topicreview/bp/ch21/gibbs.php
- http://www.science.uwaterloo.ca/~cchieh/cact/c123/chmkntcs.html
- https://study.com/academy/lesson/balanced-chemical-equation-definition-examples.html