Electromagnetic spectrum (Basic terms)
Hi, Steemians! In today's post is focused on the study of the electromagnetic spectrum, which consists and what are the rays that form it. Of the four fundamental forces that we know so far (gravitational, electromagnetic (EM), strong nuclear and weak nuclear) our life in the world of macroscopic dimensions, from millimeters to kilometers, is governed by the first two. All forms of mechanical energy are actually manifestations of MS: the fact that I can "touch" and push the keyboard keys with which I am writing this text is because the electrons of the molecules that make up the key and the electrons of my fingers are repulsed by having the same electrical charge. The force of the wind in your face and in the blades of the wind turbines, the strength of the water in the dams, even the chemical energy released in the combustion is due to the EM interaction. The weak and strong force have effects only on the dimensions of the atomic nuclei.
Source
Therefore, it is inevitable that in any discussion about the macroscopic world the EM spectrum will be treated sooner or later. Especially in astrophysics the knowledge of this spectrum is indispensable, since very many of the information we receive from the cosmos is through the EM radiation - from the light of the stars, of the galaxies, of the interstellar clouds, of the jets of black holes ... But: What is a spectrum? So let's see some basic principles below.
The electromagnetic spectrum: definition
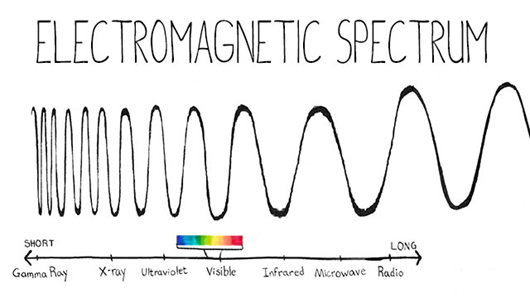
We call the electromagnetic spectrum the set consisting of waves, from those that have greater length (for example, radio waves or waves that belong to sounds that can be perceived by the ear of the human being) to waves of shorter length (for example , gamma rays or cosmic rays).
Source
The fundamental characteristics to study and describe the different types of waves are, apart from the wavelength; the energy and the frequency.
We must take into account the higher the wavelength, the lower the frequency, and the lower the wavelength, the higher the frequency.
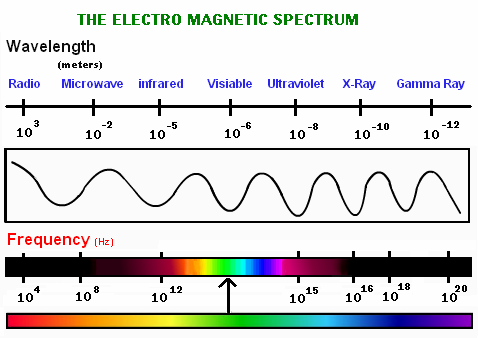
From the previous observation, we can also give an analogous definition for the electromagnetic spectrum by referring to the frequency of the waves.
Source
When we refer to an object, we call the electromagnetic spectrum electromagnetic radiation that can absorb or emit said substance. Spectroscopes are used to observe the spectra, which also allow us to measure the wavelength, as well as the frequency and energy (or intensity of the radiation).
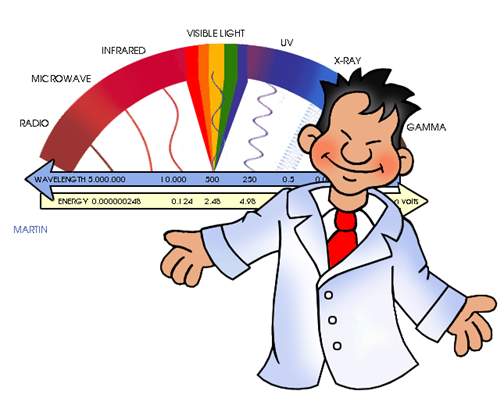
The waves that form the electromagnetic spectrum and that we can observe in the following image are (describing them from those with shorter wavelength to those of greater):
Gamma Rays: These are the waves that have the shortest wavelength, and therefore, the highest frequency. The’re the most penetrating waves known, also have high energy that allows them to travel very long distances through the air.
X-rays: These are located between Gamma rays and ultraviolet rays, therefore they have more energy than ultraviolet rays. They are used in a large number of industrial and scientific applications. Emphasizes, above all, its use in medicine where the use of radiography is very important. In spite of everything, they can be very dangerous, since they consist of a form of ionizing radiation that emit the electrons that are in the outside of the nucleus.
Ultraviolet rays (UV): These waves are in the frequency range between 7.5 × 10 ^ 14 Hz and 3.0 × 10 ^ 16 Hz. The best known types are UV-A rays (ultraviolet-A) and UV-B (ultraviolet-B). Many of the rays of the Sun that the Earth receives, and also some that provide certain rays lamps; They are of the UV-A type, so it is dangerous to expose yourself to them in excess since they can appear skin cancer. Although it is also true that if exposure to them is moderate, they favor the creation of vitamin D. But as we already know, the ozone layer helps us to protect ourselves from these rays by acting as a natural filter.
Light visible (or visible spectrum): It is the part of the electromagnetic spectrum that the human eye is able to detect. It covers all colors: from blue at 400 nm to red at 700 nm.
Infrared (IR) (or thermal) radiation: The infrared are in the frequency range between 3.0 × 10 ^ 11 Hz to 3.8 × 10 ^ 14Hz. The vast majority of infrared rays we receive are from the Sun, although any molecule that has a temperature above 0 ° Kelvin emits infrared rays. Infrared rays are very useful for meteorology, since from a photo of the Earth from a satellite using infrared rays, you can know the temperature in each area of the Earth, depending on the different colors that appear.
Radio waves: Lastly, radio waves are those with the longest wavelength. They are used mainly for television, mobile phones and radio.
The spectra can be seen through spectroscopes that, in addition to allowing the spectrum to be observed, allow measurements to be made on it, such as the wavelength, frequency and intensity of the radiation. The length of a wave is the spatial period of a wave, that is, the distance from pulse to pulse. Frequency is a quantity that measures the number of repetitions per unit of time of any phenomenon or periodic event.
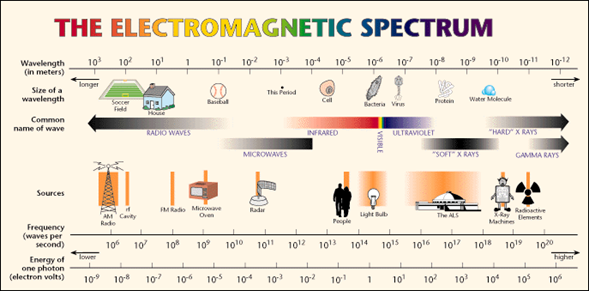
The electromagnetic spectrum extends from the radiation of shorter wavelengths, such as gamma rays and X-rays, passing through ultraviolet light, visible light and infrared rays, to electromagnetic waves of longer wavelength, such as radio waves. It is believed that the limit for the smallest possible wavelength is the Planck length while the maximum limit would be the size of the Universe although formally the electromagnetic spectrum is infinite and continuous.
For its study, the electromagnetic spectrum is divided into segments or bands, although this division is inaccurate.
Source
Much information can be obtained about the physical properties of an object through the study of its electromagnetic spectrum, either by the light emitted (black body radiation) or absorbed by it. This is spectroscopy and is widely used in astrophysics and chemistry. To do this, the emission and absorption spectra are analyzed.
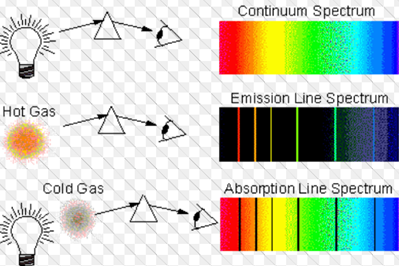
Source
The atomic emission spectrum of an element is a set of frequencies of electromagnetic
waves emitted by atoms of that element, in a gaseous state, when energy is communicated to it. The emission spectrum of each element is unique and can be used to determine if that element is part of an unknown compound.The absorption spectrum of a material shows the fraction of the incident electromagnetic radiation that a material absorbs within a range of frequencies. It is, in a sense, the opposite of an emission spectrum. Each chemical element has absorption lines at some wavelengths, a fact that is associated with the energy differences of its different atomic orbitals. In fact, the absorption spectrum is used to identify the component elements of some samples, such as liquids and gases; further, it can be used to determine the structure of organic compounds. An example of the implications of an absorption spectrum is that the object that does so with the colors blue, green and yellow will appear red when white light strikes it.
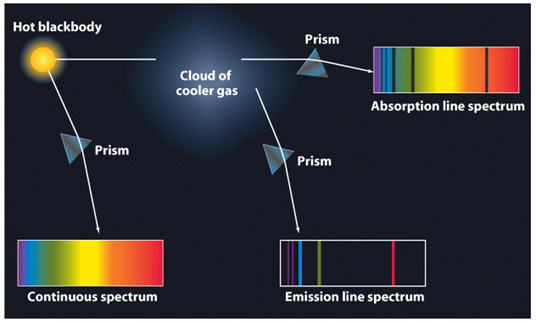
Source
In the image of the previous one we can see an example of application of the study of the spectra. When the light falls on a cloud of gas, its subsequent study reveals the components of which it is formed, since only those wavelengths that have not been absorbed by the cloud will pass. Each element has its own spectral signature.
The speed of light
The speed of electromagnetic disturbances in the vacuum is a universal constant. It is called speed of light and is indicated by C. The speed of light in vacuum is a universal constant rounded to 300,000,000 m / s (300 million meters per second), but the exact figure is 299,792,458 m / s. In other media it varies according to electromagnetic factors: for example, the light in the air moves to 299,708,000 m / s, while in the water it moves more slowly, to 224,902,000 m / s. For two thousand years it was believed that light traveled with infinite speed. Many, like Galileo, tried to measure it without success.
Ultraviolet radiation
Ultraviolet radiation is called electromagnetic energy emitted at wavelengths less than that corresponding to that visible by the human eye, but greater than that which characterizes X-rays, that is, between 100 and 360 nm. The radiation of wavelength between 100 and 200 nm is known as far ultraviolet or vacuum. Commonly comes from the sun or gas discharge lamps. Ultraviolet radiation is so energetic that its absorption by atoms and molecules produces joint ruptures and ion formation (photochemical reactions), in addition to electronic excitation. Prolonged exposure of human skin to ultraviolet rays predisposes to the development of skin cancer.
Oxygen and nitrogen in the atmosphere absorb virtually all of the far ultraviolet radiation from the sun, transforming its enormous energy into photochemical reactions and thus preventing it from reaching the earth's surface, where it would destroy complex molecules, and so it would make the existence of life so impossible.
Thank you for readme...
Greetings!
For more information visit the following links: