Nope, We Can't Invent A Perfect Engine
Pixabay image - (CCO Licensed)
We have made some improvements through these decades on how we power our society. But by in large, our world still runs on force of fuel and nonrenewable energy. And as long as we depend on these limited resources, we will need to decide on the most efficient ways to have this energy. But that is difficult because the laws of Physics are not in our favor. The fact is you cannot make super-efficient system without any waste. No matter how good of an engineer you are, you cannot invent a perfect engine. Why? It is because energy caused this to prevent our universe. And it is caused by Entropy and the Second Law of Thermodynamics.
Thermal Efficiency
Since we started designing engines, we have constantly tried to improve them. Our first engines were not that efficient, but we have come a long way since. Take for example, a Heat Engine. Simply put
A Heat Engine is a machine or system that converts heat into other forms of energy.
And we can see how efficient they are by looking at the Thermal Efficiency. The Thermal Efficiency of a Heat Engine is the amount of useful work it can produce by the amount of heat that is given. So, the more work we get out, the more efficiently we use our fuel and the less of it we need. That is why engineers are constantly trying to make engines as efficient as possible. Many of the entire combustion engines that we find on the road have around 20% Thermally Efficient. Meaning that 20% of the heat that applies to the engines actually does work.
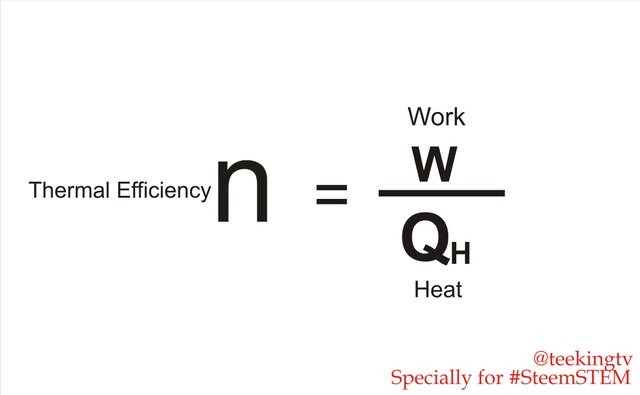 Thermal Efficiency - Made with Corel Draw.
Thermal Efficiency - Made with Corel Draw.There are some types of motor engines with 40% Efficient and there is even Acute Gas Turbine engines that delivers 61% combine in Thermal Efficiency. That is impressive but still not even close to a 100%. There seems to be major work on how efficient our engines can be. At least for now. So what gift? Why aren't they any better? Well, we are restricted by Thermodynamics.
First Law Of Thermodynamics
You see, the first law makes the whole scene pretty simple:
If we cannot create or destroy energy, then any change in energy that we have, must have an equal and opposite change in energy somewhere else.
But in reality, it is a bit more complicated than that. Based on the first law, you might think that you can simply recycle energy over and over again. But while energy cannot be created or destroyed, it can change into less useful even unusable forms. In fact, every real world energy conversion has some amount of energy that changes into a form that is unavailable to do work. And this less usable energy that is lost is usually heat.
But can't we just convert that heat back to work? Oh yes! But not entirely. Heat can never be turned into work performing type of energy with 100% efficiency. So, every time you have a transfer of energy, you end up with a more useless energy.
For example, let us say you have a hot food in a cold room. Every time the food cool off and whatever energy it releases, it is for surrounding to gain. You cannot take the energy from the cold room to heat back the food even though the exchange does not violate the first law.
Or think of it in terms of electricity running through a wire that goes through a radiator and generate heat. Electricity goes in and heat comes out. But if you try to heat the wire, you would not get electricity back even though once again, the first law is not violated. This is where the second law of Thermodynamics comes in.
Second Law Of Thermodynamics
While the first law is all about the total quantity of energy, the second law is all about the quality of energy and it states that:
As energy is transferred or transformed, more and more of it is wasted. It basically restricts the inter-conversion between heat and work.
A 100% of the work the heat present to a system can be converted into heat but only about 70% of the heat can be converted into work. To understand what this really means, let us take a look at a heat engine. An engine that converts heat into energy that does work. Let us say we have a heat energy into tyhe engine, the system will take the heat go through some process and give us energy in form of work. Some of the heat wil always be released at a cooler temperature as secondry output. Now, heat engine cannot operate without doing this.
Basically, unless we are trying to get heat, we have got to have some amount of inefficiency. So how do we find any of this out? It all goes way back to the work of two great minds. The first of these minds belongs to Sadi Carnot, the first scientist who is probably more brilliant that we will ever know. Carnot came from a famous and influential family. His father was a mathematician and a military engineer. His name would eventually be emblazed along with those of the scientists whose name is inscribed on the Heiffel Tower. And Carnot voluntarily followed his father's footsteps joining the French army chore of engineers in 1814.
But then, things got sticky. His father was helped to become the ministry of interior under fellow name Napoleon. And after Napoleon was defeated in a political war, Carnot's father was sent into exile. But Carnot was allowed to stay. He spent years inspecting army facilities and writing reports until 1819, he transferred back to Paris and out of curiosity, started attending lectures on Chemistry and Physics. Then he became especially interested in improving the performance of steam engines. He published his research in a book called Reflections On The Motive Power Of Fire in 1824. And although his work was largely ignored, it contained a revolutionary idea - A Motor For The Most Efficient Steam Engine Possible.
Carnot Cycle
Today, it has been Carnot engine. And the process by which it works is called the Carnot Cycle.
The Carnot Cycle is actually a Hypothetical process. It is the most ideal cycle of changing pressures and temperatures in a fluid.
And it is ideal because it assumed the alternate source of waste. Like friction, or conduction of heat between different parts of an engine. So we use this cycle as a standard to adjust the performance of heat engines. The Carnot Cycle consists of four processes all of which are reversible. TwoAdiabatic processes and Isothermal ones and they can take place in either a closed or steady state systems.
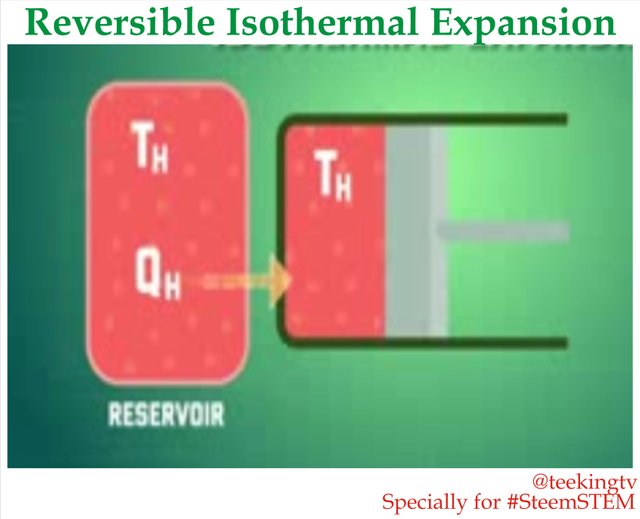
Reversible Isothermal Expansion
Let us look at the entire system. Let us say we have a gas that contained in an Adiabatic Piston cylinder system. As we start up our Piston, the first process in the Carnot Cycle is called the Reversible Isothermal Expansion. In this stage, the head of the cylinder stalls in contact with energy resource or reservoir at temperature TH. This will transfer heat which we call QH to the gas. After energy resource transfers heat, the gas also responds slowly which does work on the surroundings.
As the gas expands, the initial temperature TH tends to decrease. But as soon as this initial temperature drops by very small almost negligible amount, some heat transfer from the reservoir to the gas which brings it back our initial temperature. That means the temperature of the gas is basically kept constant throughout the process which will continue until the Piston reaches position 2.
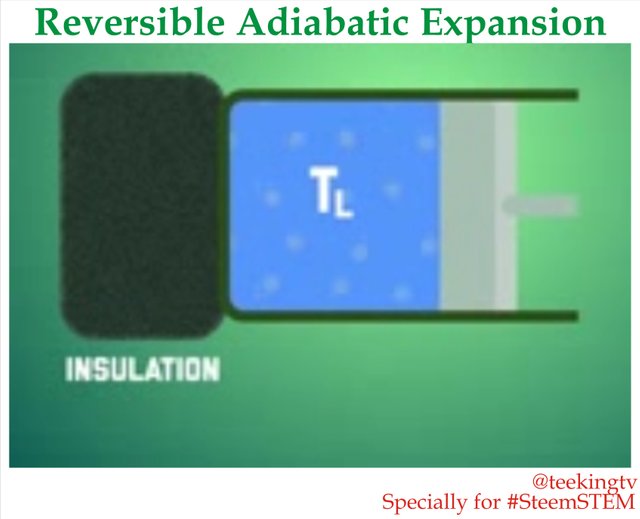
Reversible Adiabatic Expansion
At this point, we come to the second stage of the Carnot Cycle - Reversible Adiabatic Expansion.
Here, we make the process Adiabatic but replace the reservoir with Insulation. This means that as the gas expands, it can cool down since it burns the heat as backup but reservoir anymore. It will do this until the temperature drops from TH to TL which brings us to position 3.
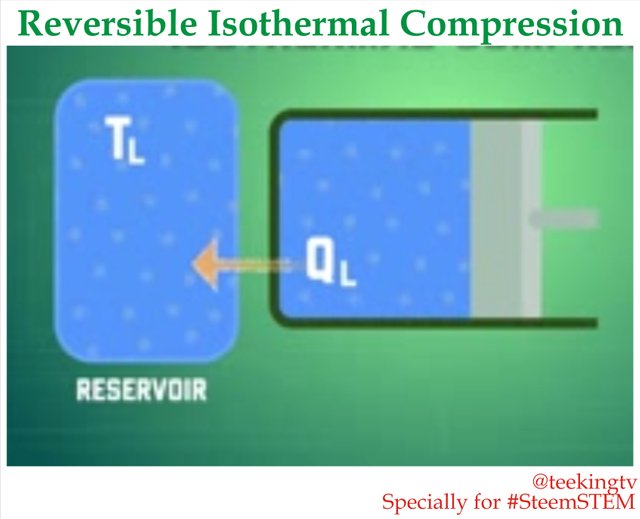
Reversible Isothermal Compression
Now we need to go in the opposite direction as we begin the third stage of our Cycle - Reversible Isothermal Compression.
So now let us remove the Insulation and bring the cylinder into contact with an energy sink at temperature TL. This will cause the gas to transfer heat which we call QL to the reservoir. Then when some external force pushes the Piston inward which does work on the gas, the gas will compress and its temperature will tend to rise. But as soon the temperature rises by a very tiny amount, some heat get transferred from the gas to the sink which cools it down causing the temperature to drop back down to TL. Now you notice that this is really similar to what happens at the first stage. Temperature of the gas will stay the same throughout the process until the Piston reaches position full.
Reversible Adiabatic Compression
Now to the fourth stage of our Cycle - Reversible Adiabatic Compression.
In this last stage, we put the Insulation back on, making the process Adiabatic again. The gas will continue to steadily compress until its temperature rises from TL to TH which will complete the Cycle and bring us back to where we started. And since we are back to where we started, we have a fully reversible Cycle. This makes the Carnot Cycle the most efficient Cycle that we can have between two different temperatures.
Now we cannot actually achieve it in reality, we can improve the efficiency of our Cycle if we try to model them more closely to Carnot design. His work showed us that the efficiency of a heat engine is only dependent of the temperatures if it is heat reservoir rather than fluid that it uses. At such, the maximum attainable efficiency of a heat engine is equal to 1 minus the temperature of the cold sink divided by the temperature of the heat reservoir.
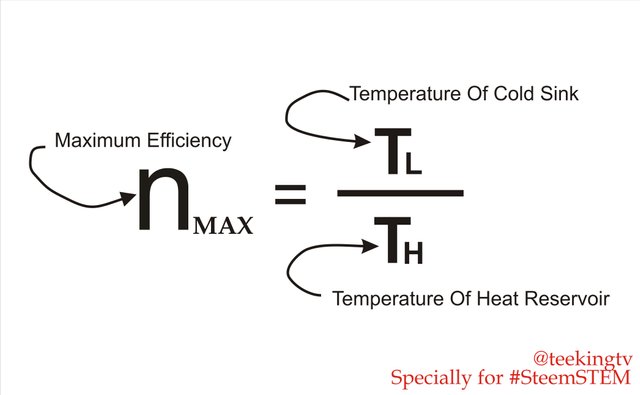
Maximum Efficiency - Made with Corel Draw
Now, remember when I said that Carnot is probably more brilliant than we will ever know? Well, let me finish his story. Soon after he published his work on Carnot Cycle, he quit his job with the army and was almost left with no income or any pension. He was then sent to asylum where he contracted cholera which was sweeping Paris at the time. Carnot died at the age of thirty six and because all of his personal belongings were considered to be contaminated, they were buried with him including all of his notebooks and papers. So the full part of his work and this genius has been lost to history.
Nonetheless, we do know that Carnot discovered the limitation of efficiency and his work has been confirmed important to our understanding of wasted energy. While Carnot insight alone did fully explain why we could never build a perfect engine, we still need to understand another property.
No one came up with new stuff until few decades after Carnot came up with his Cycle. The great man who gave us this property was Rudolf Clausius. Clausius was a German mathematician and physicist who introduced the concept of entropy around 1850 after he recognize the confusion of Carnot work and then conservation of energy.
Entropy is the measure of a system's thermal energy per unit temperature that is unavailable for doing work. It is also the measure of the disorder or randomness of a system.
Mathematically, if the energy of a system is zero, then we have a reversible process with no change in Entropy. Like with the Carnot engine, any value of zero, and then the process is reversible gains Entropy. So, every process result in no change in Entropy or increase in Entropy, it is impossible to have an over decrease in Entropy.
If you look at the system itself, it is possible to have a decrease in Entropy. But and it is very important, the Entropy of the system in the surrounding and universe will have to increase by an amount greater than or equal to the lost Entropy inside the system. Simply put universe always tends to work disorder. It is just the way with things. In fact, as scary as it may be, Entropy and the Second Law of Thermidynamics actually predict the end of the universe as we know it. If everything intends to work in this disorder, then the logical conclusoion is that all of the usable energy in the universe may one day be converted to heat. This event is known as the heat death of the universe and we have to face the fact it may one day be our fate.
Departing Thoughts
I would not worry about it because even though it makes sense in theory, many doubt if it will actually happen. But even if it did, it willl not happen for a really long time. But whether or not entropy will make the universe to come to an end, it does make it impossible to create a perfect engine with an output of a hundred per cent working energy. No matter how much effort we put in making a perfect engine, we will always have to follow the rules of the universe even if it is one that will lead to our own doom.
References
- Carnot’s Perfect Heat Engine: The Second Law of Thermodynamics Restated
- Information engine operates with nearly perfect efficiency
- Explained: The Carnot Limit | MIT News
- Heat Engine - Efficiency & Examples | [email protected]
- Heat Engine Cycle
Previous Posts
- Nah Nah I'm Not Dying Now... Immune Systems Are Natural Killers!
- Isn't The Devil In The Details And Not The Ocean? The Legend Of Bermuda Triangle
- Are We Even Intelligent Enough To Understand What Intelligence Is?
- Are We Really Humans Or Just A Bunch Of Bacteria? Too Many Misconceptions Here
- Who Says You Cannot Build Your Own Airplane? Series #11: Yo! You Don't Mess With Flight Instruments
Hey! Do you write posts that are related to Science, Technology, Engineering, and Mathematics (STEM)?
Then join #steemstem on on discord. Click here.
Check this blog post by @steemstem to understand the guidelines on how to become a member of @steemstem.
Also check on this post by @steemstem to understand the use of images so as to avoid copyright infringement.
And in case you are writing from Nigeria, you can include #stemng tag in your posts. Details on @stemng blog.
Thinking of delegating SP to @steemstem to support this great initiative? All you have to do is to use the links below:
50 SP | 100SP | 500SP | 1,000SP | 5,000SP | 10,000SP | 50,000SP
However, ensure you have at least 50 SP left in your wallet.
I am @teekingtv, the no.1 Global Meetup analyst


This post has been voted on by the steemstem curation team and voting trail.
There is more to SteemSTEM than just writing posts, check here for some more tips on being a community member. You can also join our discord here to get to know the rest of the community!
This post has been voted on by the steemstem curation team and voting trail.
There is more to SteemSTEM than just writing posts, check here for some more tips on being a community member. You can also join our discord here to get to know the rest of the community!
Hi @teekingtv!
Your post was upvoted by utopian.io in cooperation with steemstem - supporting knowledge, innovation and technological advancement on the Steem Blockchain.
Contribute to Open Source with utopian.io
Learn how to contribute on our website and join the new open source economy.
Want to chat? Join the Utopian Community on Discord https://discord.gg/h52nFrV
Nice one bro... thermodynamics is a really interesting science and i like how you have been able to use it explain this concept.