Artisanal Soaps: "Ignorance is like blindness"

Image Source: Personal.
During this last decade in Venezuela - due to the socio-economic situation developed - there has been a deficit in the normal supply of the essential products for the average person. Without referring to political issues, the level of industrial production has declined, and illicit activities further reduce the availability of these products.
In this way, a large number of individuals, based on Web articles, family traditions ... that is, erroneous methods and knowledge (except for a small group that practices their trade correctly), started a homemade production of the mentioned articles with a second mercantilist purpose, among them: flour, cheese, edible vegetable oil ... and mainly: cosmetic soap.
The production method is based on the saponification process; organic reaction where a strong base such as Sodium or Potassium Hydroxide, and a fatty acid or a mixture of them in the form of animal fat or vegetable oil are used to produce an ionic salt, and water:
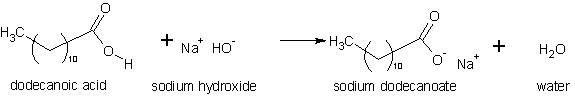
Image Source: Personal.
In this example, Lauric acid (Dodecanoic according to IUPAC) is neutralized to Sodium Laurate and water. The saponification reaction is slow, and is usually incomplete or requires a long period and heating to complete it. This is due to the apolar character of the carbon chain (C12), which must be involved in the polar reaction of the carboxyl group (COOH).
The fatty acids present in fats are found in the form of triglycerides, substances in which three carboxyl groups of the respective acid molecule are bound (esterified) with 1,2,3 propanetriol (Glycerin). Forming esters of the type: Tri-dodecanoate of 1, 2,3-propanetriyl. The fatty acids present in animal fats are generally Lauric (C12), Myristic (C14), Palmitic (C16) and Stearic (C18) acids, whose reactions are progressively slower due to the increasing length of the carbon chain.
That said, when making a cosmetic soap, it must be taken into account that the reaction is not immediate, and the soap must be left to rest for at least a month to stabilize the pH and allow the reaction to be finished. Similarly, stoichiometric quantities and technical grade reagents should be used as a minimum.
In Venezuela, instead, chemical pipeline unclogger are used as a source of hydroxide, and burned cooking oil , even with the remains of fried foods ... as a source of fatty acid. Likewise, adequate amounts are not used; and in order to send them to the market as soon as possible, they are only allowed to stand for a week at most, being used by people who do not know their origin and quality.
The effect of the resulting soap on human skin is a "daily body treatment" with sodium hydroxide. A strong base such as sodium hydroxide, still diluted, produces an elevation of cutaneous and mucosal pH with its characteristic consequences. Among them, irritation, burns, desquamation, dryness, cracks and sensitivity to environmental or external agents that under normal conditions are inhibited by healthy skin and its protective fat barrier. In this way, bacteria, UV radiation, fungi, and all kinds of opportunistic pathogens (Staphylococcus aureus from the nostrils, Escherichia coli from the genital area ...) can attack the organ that tries to repair the damage caused by the repeated application of Soap.
A sample of handmade soap of 60gr aprox. available in the market, prepared with "animal butter" and "caustic soda" by an informal businessman.
A sample of soap was taken and a qualitative analysis of the presence of the hydroxide ion was carried out. For this, a scraping of the soap was mixed with ammonium chloride solution (NH4Cl), perceiving the characteristic smell of ammonia (NH3 / NH4OH), according to the reaction:
Subsequently, in order to extract the hydroxide, the 60 g soap bar was dissolved in 250 ml of distilled water at 78 ° C, at which point it melted. It was allowed to cool and the solution was filtered. The process was repeated twice, the filtrates were diluted to 1 liter. A 100 ml aliquot was taken and it was titrated with Standard 0.01 N Hydrochloric Acid, with phenolphthalein 1% as indicator of the final point. The concentration of Sodium Hydroxide obtained was 0.00752 N, requiring 75.2 ml of acid. Calculating the pH:
The pH obtained was confirmed with an OHAUS pH-meter Starter 300, this being 11.79 (Error ± 0.03) corresponding to 0.3 grams of Free Sodium Hydroxide for each 60 g of soap bar. It should be noted that this pH is even higher than that of laundry detergents. It could be compared with the pH of the Household Ammonia (pH = 12), or of the Sodium Hypochlorite in solution (pH = 11.5).
With these results, the damaging effect of artisanal soaps WRONGLY ELABORATED on the Venezuelan´s skin is confirmed, a country where large amounts of UV radiation are received, with the most prone conditions for premature aging and the development of cutaneous diseases.
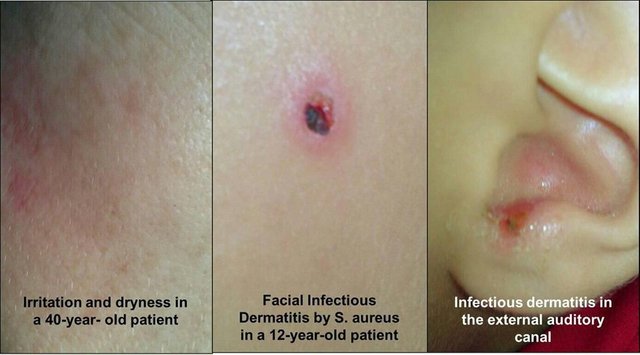
Image Source: Personal.
Regards!!
References:
• Mortimer, Charles (1983) Ed. Iberoamérica
• Química Analitica Cuantitativa. Ed. Kapelusz. Vol.1, A.I. Vogel. 3ra Ed. 1960.
• El Manual Merck. 10ma Ed. 1999.
• Fitzpatrick. Atlas De Dermatología Clínica. 7ma Ed. McGraw-Hill EDICIONES. 2014
Very informative and intelligent

Thank you for your contributions!
Good fortune.
Hi, thanks for your comment, it's a pleasure!
I gave you my first resteem.

Your blog has great information to disseminate.
Thank your for shareing your knowledge
Hi, Thanks for comment!
We should work together
We create a community so that we are united in it, I have created a WHATSAP group , you can come from it or give your WhatsApp number.
Join link
https://chat.whatsapp.com/4mvFHrrT4o2Em52IhdrGCl
Hello! great!
Join us click here link our group
https://chat.whatsapp.com/4mvFHrrT4o2Em52IhdrGCl
U are beautiful
Thanks a lot! Regards!:)
Obsessed with ur knoweldege post that makes me followed uh and upvote it will be great help if you follow back and will help me growing over this steemit platform waiting for some more informative posts
Hi! sure I will follow you, thanks for your comment!
https://steemit.com/polish/@laptopy2005/surwiwal-of-the-21st-century-history-which-scenario-have-written-life