Quantum-mechanical Carnot-like engine: Gas from states belonging to the well of infinite potential
Quantum-mechanical Carnot-like engine
For centuries, scientists have tried to find, or propose cycles of thermodynamic machines that have an efficiency closer to that of Carnot, in their attempts, multiple cycles have been proposed, among these we have a cycle based on the laws of mechanics quantum, that is, one in which the working substance is the state gas of the quantum mechanical particle.
Here discussed similarity between quantum mechanics and thermodynamics, an analysis of the antecedents of the quantum Carnot cycle is exposed.
In the year 2000 C. Bender[1] publish an article titled “Quantum-Mechanical Carnot Engine” in which he exposes the bases for the creation of a Carnot machine and applies them to a gas of quantum states of a particle belonging to the infinite square potential system, later S. Abe[2] publish an article titled “General Formula for the Efficiency of Quantum-Mechanical Analog of the Carnot Engine” which exposes a general formula to calculate the efficiency of Carnot, however said formula has certain limitations. These two articles are discussed here.
Quantum processes for a Carnot Engine.
Let F be the force that applies the gas of quantum states on the well wall, causing it to expand. The force is defined as
Processes quantum adiabatic.
An adiabatic process is one in which the system is thermally isolated, classically. An adiabatic expansion is defined by the first law of thermodynamics as , that is, the variation of heat is zero. In is processes the internal energy of the gas is converted into mechanical work. In an adiabatic process, the size the potential well changes as the wall moves, so that the system is kept in thermal equilibrium all the time, as the absolute values of the expansion coefficients |an| must remain constant, this means that the process has infinitesimal variations. Not expect any transitions between states to occur during an adiabatic process, however, it is clear that as L changes, the eigenstates φ(x) and corresponding energy eigenvalues En vary according to L.[1] The characteristic equation of an adiabatic process is
Processes quantum Isothermal.
During this process the system is in contact with a heat source so that the temperature T of the gas in the cylinder remains fixed. As the piston moves, the system does work. However, since the temperature of the gas remains constant, the internal energy of the gas also remains constant. The initial state of the system φ(x) with a volume L is a linear combination of its eigenstates. The expectation value of the Hamiltonian remains constant as the size of the well potential changes.[1] The characteristic equation of an isothermal process is
Infinite One-Dimensional Potential Well
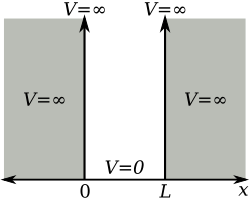
Let us consider a particle of mass m in one - dimensional box of width L . The time - independent Schrödinger equation for this system is
A solution of the form is proposed. Solving and applying the border conditions
, we have
And eigenenergy
Quantum-mechanical Carnot-like engine.
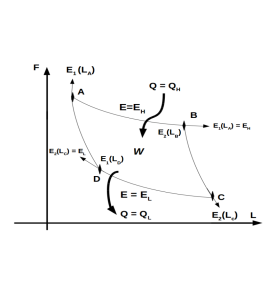
We will only work with the first two energy levels. This is
with
Using the quantum adiabatic process and the quantum isothermal process defined above, we have that the forces in each of the processes is
Processes Isothermal: Expansion
At this point the system is so that
As in an isothermal process, the internal energy remains constant, that is to say
so the system expanded
to reach the second state.
Processes Adiabatic: Expansion
At this point, the system is in , and has a force of the form
Processes Isothermal: Compression
Here the system is in . So the force is defined as
And . The system compresses the inverse of its expansion, as one would expect from a reversible cycle. This is
Processes Adiabatic: Compression
In this process that finishes the cycle, the system is in .So the force is defined as
Total work of the cycle.
The mechanical work W done in a single cycle of the quantum heat engine can be evaluate with the following integrals:
Solving the integrals, we have
Energy absorbed by the cycle.
Then, the energy absorbed by the potential well during the isothermal expansion is
Resolving, we have
Efficiency
The efficiency η of our two-state quantum heat engine, as given in the general formula
Substituting, we have
And this is
The general result, is an efficiency that depends on the initial and final energy or temperature. This result makes a general confirmation that for the higher state QHE the work to be looser than that for lower sate system under certain condition. It is concluded that the efficiency of a Carnot quantum cycle is similar to the classic Carnot efficiency, only that in the quantum case the temperatures are substituted by the first two energy levels, the lower energy level corresponding to the lowest temperature, and the highest energy level at the highest temperature. It can also be concluded that this formula of efficiency does not work for cases where the energy spectrum is non-homogeneous. And in this case, where the energies present these forms, it also happens that the characteristic equations for each process are not met
For more information consult the bibliography:
[1]. Bender C., Dorje C., Bernhard K. (2000) ”Quantum-Mechanical Carnot Engine” Journal of Physics A: Math. Gen. Vol. 33 N. 24, p.(4427–4436).
[2]. Sumiyoshi Abe (2013) ”Quantum-mechanical analog of the Carnot cycle: General formula for efficiency and maximum-power output” 12th Joint European Thermodynamics Conference Brescia, p(334-336).
Congratulations! This post has been upvoted from the communal account, @minnowsupport, by sneikder from the Minnow Support Project. It's a witness project run by aggroed, ausbitbank, teamsteem, theprophet0, someguy123, neoxian, followbtcnews/crimsonclad, and netuoso. The goal is to help Steemit grow by supporting Minnows and creating a social network. Please find us in the Peace, Abundance, and Liberty Network (PALnet) Discord Channel. It's a completely public and open space to all members of the Steemit community who voluntarily choose to be there.
If you would like to delegate to the Minnow Support Project you can do so by clicking on the following links: 50SP, 100SP, 250SP, 500SP, 1000SP, 5000SP. Be sure to leave at least 50SP undelegated on your account.
I support you with my resteem service
Your post has been resteemed to my 3300 followers
Resteem a post for free here
Power Resteem Service - The powerhouse for free resteems, paid resteems, random resteems
I am not a bot. Upvote this comment if you like this service
thank you for this post
please help me i need some upvote @pkalmik
Well done! This post has received a 5.00 % upvote from @litasio thanks to: @steemstem-bot. Whoop!
If you would like to delegate to the @LitasIO you can do so by clicking on the following link: 10SP