Oxygen: NOBODY SEEMS TO CARE, APPARENTLY

Everybody has heard of oxygen, more commonly know as the "thing" which is the basis of all life on our planet. And sometimes, a "thing" for conspiracy theorists how it slowly poisons us. Amazing world we live in. But limited to only as the thing we living beings need to survive. Before i start my gibberish "thing", this "thing" can make you high ;). Haha. Since, I have your attention now let us dig a lil' deep into it.
AND I HAVE INSERTED A RAP ABOUT OXYGEN SOMEWHERE IN BETWEEN :) AND ALSO A SMALL CONTEST/QUESTION FOR THE GENERAL POPULATION AT THE END.
Formed at least 13.1 billion years ago, third most abundant element in the universe by mass (triggered conspiracy theorists), after helium and hydrogen, member of group 16 (no, not a boy band) or VI A, period 2 of the periodic table, the one and only, free air (WHAT?), the dephlogisticated air (the what?), the OXY-GEN ( finally, the one i've heard). What did i mean by this, some of you might be wandering. Just pointed out the fact that our beloved oxygen was not always named oxygen (pewdiepies' voice). WHY? Because oxygen is a cheater! Discovered by Carl Wilhelm Scheele in 1773 or maybe earlier and by Joseph Priestly in 1774, separately. (Gets Discovered by just anybody ;), you could discover it too and name it as you please, and the way to discover the gas-whore, the ways are here somewhere, You just have to find it and name it. And also i have not specified the gender of the gas-whore, not a misandrist or a misogynist). Although, Carl wilhelm scheele discovered it prior, Joseph priestly is given the credit because of his early published work, (Life, eh?). Scheele discovered the "FREE AIR" by heating mercuric oxide(HgO) and various nitrates, whereas Priestly focused sunlight on Mercuric oxide inside a glass tube, which liberated the "DEPHLOGISTICATED AIR." (So, who named the gas the what has been cleared). So, who gave the name "oxygen". Who have we missed? Antoine Laurent Lavoisier. First he named it vital air, and later changed it to oxygène in 1777, which can be broken down into oxy(acid in greek) and genes(producer). WHY? because he mistakenly believed that oxyen was a constituent of all acids. Even though the idea behind was wrong but the name remained. With this, we've completed the H-I-S-T-O-R-Y section.
With a A++ in history section, without any further a-due, let me talk about the type of element oxygen is and also it's properties. Since it is chemically reactive it's never found in it's atomic state and is found in free state in air as O2, dioxygen, where two oxygen atoms are chemically bound to each other. This is the form of oxygen that we all living beings use, OXYGEN GIVETH AND TAKETH THE LIFE AWAY.
PROPERTIES SECTION

SYMBOL: O
ELECTRONIC CONFIGURATION: 1s22s22p4 OR [He]2s22p4
ATOMIC NUMBER (Z): 8
ATOMIC WEIGHT: 16 (15.999)
STATE: 1) GAS: COLORLESS 2) LIQUID: PALE BLUE
ALLOTROPES: O2, O3(THE GOOD GUY OZONE)
PHYSICAL PROPERTIES OF OXYGEN FOUND IN ATMOSPHERE
PHASE (AT STP, STANDARD TEMPERATURE AND PRESSURE): GAS
MELTING POINT: -218.90C (-363.820 F (IMPERIAL THUGS))
BOILING POINT: -182.9620C (-297.3320F)
DENSITY AT STP: 1.429 g/L
PRESENCE IN ATMOSPHERE: 21% BY VOLUME AND 23% BY WEIGHT (IN O2 FORM)
ENTHALPY OF FORMATION[H]: (Positive meaning, energy is required)
ATOMIC OXYGEN (O) : 249 kJ/mol
OXYGEN OR DIOXYGEN (O2): 0 kJ/mol
OZONE (O3): 143 kJ/mol
We already found that its' presence is huge(Uge, ;)). With about 50% of mass inside the earths' crispy and hot crust, 80% by weight in water and about 50-70% in plant and animal tissues. I mean, look at the numbers, it was no mere coincidence that it was discovered by so many people.( OR DID IT LET ITSELF TO BE FOUND OUT? WE"LL NEVER KNOW ). And in combined form with metals and nonmetals to form oxides, basic or acidic. Gets along with just almost everybody.
Also, found in other states which are known as the allotropes of oxygen. (WHAT IS THE DEAL WITH THIS OXYGEN! GAS WHORE CONFIRMED). So, before we discuss about these, first, what is allotropy? Dictionary.com defines it as "one of two or more existing forms of an element. Eg: Diamond and graphite are two allotropes of carbon." Already learned that there are two allotropes of oxygen in the properties section. Diatomic or dioxygen or the which we normal people call oxygen, and triatomic or the good guy ozone.( Before anything else, WHAT IS ALL THIS FUSS WITH OZONE, OZONE IS REAL YOU FAKE PEOPLE)
We've almost discussed everything about the DIOXYGEN (O2). Most common allotrope of elemental oxygen on earth, also known as molecular oxygen and more commonly know as oxygen.
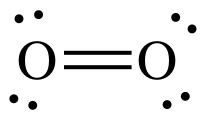
With a bond length of 121 pm (picometer) and a bond energy of 498 kJ/mol. The properties are mentioned above in the properties section of oxygen found in the atmosphere.
OZONE (O3)
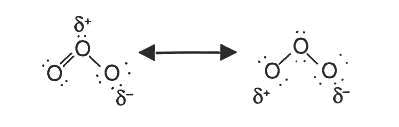
Ozone needs its own heading because it has been subjected to harsh conditions by us, humans. The triatomic state of elemental oxygen, which is much less stable than the diatomic allotrope. It is formed in the stratosphere region when UV light and other electrical discharges present hit the diatomic oxygen. With the higher concentration region lying in the ozone layer of the atmosphere, which is responsible for abosrbing most of the UV radiation from the sun, which otherwise would have caused skin cancer.
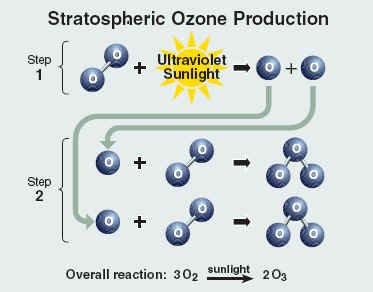
BEFORE IT STARTS GETTING TOO MONOTONOUS, THE RAP IS NOT THAT FAR AWAY.
Because of depletion of this layer, we have banned the use of CFCs and related hydrocarbons. And recently in news, we heard that the depleted layer is slowly recovering, a good news for us. We are not dying of cancer. HURRAY! If you're interested in this then here's a LINK how the scientists involved found out that it's healing itself. (PHOENIX OF SOME KIND)
FINALLY, THE RAP SECTION, PUT SOME BEAT TO IT
You need it for combustion to burn the whole crew.
but the atomic form is too simple though
But don't underestimate the power of the symbol O
It makes zone save you from burns that are savage
Life wouldn't be possible without it that's established
Family of Calcogens, also called ore formers
In all corners, like a known genre
And you know who's fine
someone who's got oxygen, in the system, its not really toxic and
density as thin as 1. four two nine
I be an addict sniffing it day and night
Staying tight, taking it with me to them greatest heights
it's colorless and odorless and tasteless
Kind'a like today's music, but there's a difference, I don't hate it
I rhyme for this shit, Even if I die I'll survive for this shit
Now the part, we all have been waiting for. Section where you get to name the gas anything you like.
There are many ways to produce oxygen gas in the lab, and when i was in high school, this is the procedure that we adopted. I did not have cell phone during the time so had to import the picture from someone elses' site.
In the ratio of 4:1, the mixture of powdered potassium chlorate (KClO3) and manganese dioxide(MnO2) is heated at 200-3000C, in a hard glass test tube. The reaction is as follows:
There you go, it wasn't that hard, was it? Just buy the above said things and let yourself discover the "what-ever you want to name it" gas. (Name it love, and say i can't live without you love or something else.)
I am starting to think that the post has been dragged for too long. HAHA. Don't worry only 5 or more paragraphs to go ;). (ALSO I HAVE AN EXPERIMENT FOR YOU GUYS TO PERFORM AT HOME)
Started this gibberish thing a long time ago, but what is the significance of this? Let's find that in the USE section.
USES
- Surviving, duh! I mean, you can't miss this. Oxygen is what that gets attached with hemoglobin of the blood and goes on a roll round the body. Them tissues and cells need oxygen.
- Angry with someone, need to burn down their house, make sure that oxygen is available in the place. As Oxygen is a must for combustion. If the said person somehow manages to create a vacuum around his/her house, your plans gonna fail. IMPORTANT POINT.
- Lighting a fire on someone's ass. haha. Particularly meant for Rockets. Three things required for combustion are Oxygen, heat and fuel and without oxygen, no man would have landed on the moon.
- Oxy-Acetylene Welding anyone? The same concept as stated above. Why? A feciliator for combustion.
- When doing space walk, duh! point 1 again.
- Treatment of wastewater. As someone who worked as an intern for a month in one of the multinational compnay, oxygen is lacking in these wastewaters. Dissolved oxygen is required and is added to maintain the BOD/COD (Biological Oxygen Demand/ Chemical Oxygen Demand)
- OZONE! HOW CAN WE FORGET THIS. Basically don't use it directly but someone above is watching us and protecting us from CANCER. Do not take everything for granted.
NOW THE EXPERIMENT THAT ANYONE CAN PERFORM AT HOME
I stated many times, that oxygen is needed for combustion and this experiment is to verify the said statement.
So, take out your candles, that you've been saving forever for you candle light dinner. Never gonna happaen. You'll stay single forever.
STEP 1:

STEP 2:

Take the glass and partially cover the candle and boom, it's magic. The brightness decreases suddenly. Why? A question for you.
STEP 3:

Now, cover the candle completely with the glass. Flame out. This happens because there is no oxygen inside the closed space.
STEP 4:
But, many of you at this point might be wandering, Wait a minute, this doesn't prove that it's because of absence of oxygen! Well, i understand your confusion. And i have a solution for it. You probably can buy oxygen cans/cylinder in your country. Cut a hole at the side of the glass and supply the oxygen while the candle's covered. And i guarantee that the flame will keep on shining on and even much brightly, because in a small space, lot more amount of oxygen is being pumped in.
Since, in our country those oxygen gas cans are not easily available and even if they are they cost too much for a bloke like me. So, no photo for this section.
Before i conclude my post, One last section! I swear. And this might just be the section that you've all been waiting for.
Getting high on Oxygen
It's not like weed or anything but a high flow of oxygen can make you feel relaxed, can relieve your pain and stress, reducing anxiety. In the long run, it can do some harm but them oxygen can't kill you.
References
- https://en.wikipedia.org/wiki/Oxygen
- http://www.rsc.org/periodic-table/element/8/oxygen
- https://www.britannica.com/science/oxygen
- http://letslearnnepal.com/class-11/chemistry/inorganic-chemistry/oxygen/laboratory-preparation-of-oxygen/
- https://skeptics.stackexchange.com/questions/3022/does-oxygen-get-you-high-enough-to-keep-you-calm-during-a-plane-crash
- https://sciencing.com/10-uses-oxygen-8634456.html
- https://www.scientificamerican.com/article/origin-of-oxygen-in-atmosphere/
- Chemistry books shown above.
CONTEST
Here's a contest for the general people and i plead the members of the science community to refrain from answering. The intention is to create a scientific awareness among the general population.
Let's take your cell-phone for example. The mass of the cell phone, for the sake of saying, is 2 kg measured at earth, where the acceleration due to gravity(g) is 9.81 m/s2. I want you to comment down below the mass of the cell-phone measured at moon, where the value of g is 1/6 th of the earths value and also the mass of the cell-phone at mars, where the value of g is 1/3 rd of the earths value.
RULES:
There are no rules except don't comment twice, no editing of the comments.
PRIZE:
My journey has been ok so far on steemit. Haven't made a lot but the person to answer first will receive 1 SBD ($6, approx). AND YES I DO HAVE 1 SBD IN MY WALLET.
P.S.1 This is me returning to my forte. A student in the field of applied science (Engineering), which is a branch of pure science. Previously, i have put out contents that were related to my hobby: photography and sketches, but science is what I'm all about. This doesn't mean i am stopping my photos and sketches.
P.S.2 Would love to hear your suggestions and comments on my shortcomings, whether or not if you liked the post, and if you did not, please point out the places where i need to improve. Will try to meet them in the next post as much as i can. Thank you for bearing till now. :)
The mass will not change, because it only measures the amount of matter something contains. It will be 2kg on all planets.
Looks like you're the only one.
Awesome! When will i receive my prize? I really enjoy your blog btw.
Thanks man. Let me surprise you. Haha
Are you serious about the prize or was it just a scam?
You didn't quite get me. What i meant was, this post has still 5 days to it and when you asked about recieving the prize, i said let me surprise you.
One of these(five) days you're gonna see 1 SBD in you wallet. Just wanted to surprise you.
Hhaha. Hope this clears your confusion.
Oh great haha sorry didn't understand, I'm a complete newbie
But, now since the plan's out there, not going to be a surprise, is it?