The quest for the elixir of life - Episode 4 - Tackling immunosenescence
The immune cells - the superheroes in our body fight the invaders. But of course to fight they need to eat. Like any other cell in the body, they get nutrients from the food we eat. Cells including immune cells respond to nutrients or their lack. But how do immune cells choose to respond to it? Do they age gracefully in a lower energy supply? In this episode, we will explore the effect of caloric restriction on immune cells and how it slows down ageing if it does.
Illustrated by @scienceblocks using following images -
neutrophil by Blausen.com staff (2014). "Medical gallery of Blausen Medical 2014". WikiJournal of Medicine 1 (2). DOI:10.15347/wjm/2014.010. ISSN 2002-4436. | CC by 3.0
Age youth contrast by Geralt. | Pixabay
Connective tissue by Berkshire Community College Bioscience Image Library | Public Domain
Recap
Till this point in the series we saw - how DNA damage and perturbed DNA repair pathways contribute to ageing (Also see this blog). We also saw how mitochondria, the cell organelle that derives energy out of food using oxygen, also contributes to the process of ageing. The mitochondria become less efficient as we age. They produce more ROS and contribute to cellular damage. Its biogenesis and fission and fusion dynamics also get perturbed. It also affects cell renewal via stem cells. We also saw that caloric restriction can slow down ageing. We saw that cutting off a few calories from the diet causes the cells to sense the lack of nutrition. Caloric restriction activates/inactivate different pathways - including but not limited to mTOR, AMPK, and sirtuins which then affects the stuff mentioned above - it increases DNA repair, decreases damage caused by mitochondria, and even improves mitochondrial morphogenesis. In our quest, we also explored certain molecules that can mimic the effect of caloric restriction. However, we knew that we still don't understand the complete story of ageing. Hence, we dug further.
In the most recent episode, we shifted our focus from damage that arises from within the cells to that which arise from outside. We talked about the chronic low-grade inflammation that increases with ageing. We also tried to dwell on possible reasons behind it. We talked about the damaged molecules and senescent cells that increase with age and tend to recruit more inflammation-causing immune cells. We also realized that the immune system itself ages. We saw that the immune cells differentiation from hematopoietic stem cells is affected. The way they migrate, the receptors express and things they secrete are all prone to the course of ageing. However, what we did not do is look at how caloric restriction affects this ageing immune system and inflammation if it does. In this episode, this is what we are going to explore and figure out some more elixirs of life.
A Tale of Inflammation and Caloric Restriction.
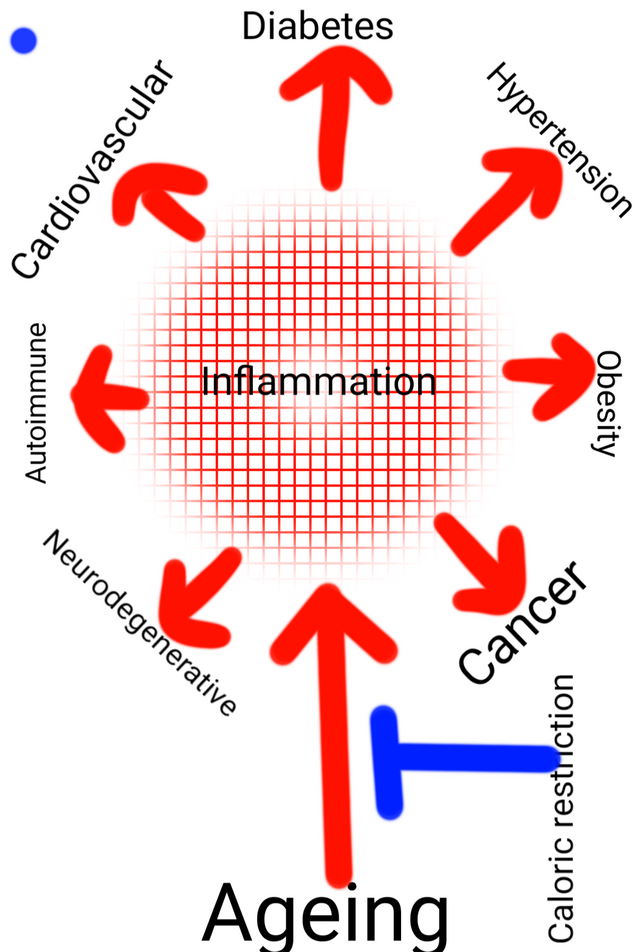
Illustrated by @scienceblocks
What we know is that many age-associated diseases also have an inflammation component. Hence, to know whether the caloric restriction has any effect on improving inflammation status, we should ask what happens to these diseases on a calorie-restricted diet. Caloric restriction has been shown to have protective effect or delay the onset of the chronic inflammatory diseases - such as type 2 diabetes, hypertension, osteoarthritis, cardiovascular diseases, and even cancer (Radakovich et al., 2019, Omodei et al., 2011).
Also, since autoimmune diseases are classic examples of inflammation gone haywire, and their incidence increases with age - it would be interesting to ask if caloric restriction does play a role in soothing them or delaying them. In 1992, Kubo et al.,, fed NZB mice (a mice model for autoimmune disease development) with a calorie-restricted diet. They observed that it prevented the onset of autoimmunity in these mice - it normalized the T and B cell number. It normalized the size of the spleen and also had an effect on non-B and non-T lymphoid cell numbers. Then in 2016, Choi et al.,, tested a fast mimicking diet in mice model for multiple sclerosis. They found that this diet was helpful not only in ameliorating the symptoms of the disease but also in reversing some of the damage. Moreover, they also did a small trial on human patients and got some exciting preliminary results.
Though, it was observed in these studies that at least a part of this effect is mediated via apoptosis of autoimmune T and B cells. However, what is not clear is that if it was a direct effect on ageing phenotype of the immune system or indirect effect on some other cells. In order to know this, we need to see if the caloric restriction has any effect on infammgeing sources that we discussed earlier. Let's see, shall we?
Does calorie restriction counter Immunosenescence?
What happens to hungry T cells?

Modified from a T cell cartoon by Blausen.com staff (2014). "Medical gallery of Blausen Medical 2014". WikiJournal of Medicine 1 (2). DOI:10.15347/wjm/2014.010. ISSN 2002-4436. | CC BY 3.0
In the last blog, we saw that the immune system gets old as well - aka immunosenescence. The innate immune cells overpower adaptive immune cells. Well, because there is a decrease in the number of naive T cells and B cells. However old senescent T cells which have short telomeres and reduced proliferation capacity accumulate. The environment of thymus, where T cells differentiate also changes (Duggal, 2018) - Thymus is where T cells mature, where its decided if they will be helper T cells or cytotoxic T cells, where they learn the difference between self and foreign, where they trained to be tolerant or offensive against certain molecular patterns on other cells and pathogens. Now just imagine the dread of the immune system if this sacred organ changes with time. As if getting new lymphocytes(T and B cells) from bone marrow was difficult enough in the old body, now we also have issues in an organ where they mature.
.jpeg)
Image by Openstax college | CC BY 3.0.
Nevertheless, it has been observed that thymic adipogenesis (one of the phenotypes associated with ageing thymus) is reduced by caloric restriction. The caloric restricted mice show more intact and functional thymus, high expression of IL2, and high proliferation of T cells as compared to their age-matched controls (Yang et al 2009). Furthermore, the caloric restriction also seems to take care of old T cells. Spaulding et al., 1997 showed that the old mice are worse at getting rid of damaged T cells via irradiation, heat shock or chemical damage. However, the caloric restriction made them efficient at it. And not only caloric restriction made them efficient at removing damaged T cells but it also inhibited them from becoming senescent, to begin with (Messaoudi et al., 2006). Plus that increased IL2 in the thymus, that helps regulate metabolic profile of T cells and promote T regulatory cell development (T reg cells helps put a break on other T cells when their job is done). Moreover, in mice on a calorie-restricted diet it appears that last stage advanced differentiation of T cells and even natural killer (NK) cells are inhibited (White et al., 2017). This kind of keeps the pool of naive cells ready, while at the same time inhibiting cynical old guys to survive too long and cause damage (which may even prevent any age-associated self-destruction).
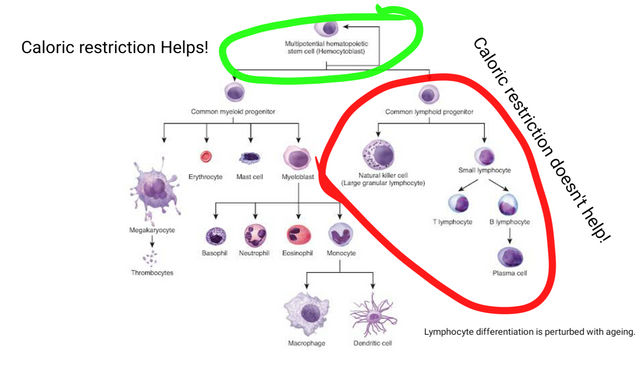
Adapted from Hematopoiesis by OpenStax | CC BY 4.0
However, despite all the good effects of caloric restriction it does not seem to have any benefit on rescuing lymphoid differentiation of HSCs, that we saw in the last blog is also impaired with ageing. It kind of slows down the ageing of hematopoietic stem cells by keeping them in a quiescent state. At first look, this may seem good, but if you think about it then if HSCs don't respond adequately to pathogen and other stressors this might be dangerous outside sterile conditions of the lab (Tang et al., 2016).
This may indicate three things - one being that caloric restriction is not sufficient to reverse all the effects of ageing and might need supplementation via other nutrients or drug to make it more optimal. Second, that rescue of autoimmune disease symptoms might be limited to caloric restriction killing autoimmune lymphoid cells and third that CR might also mellow down cells of the innate immune system which causes some of the damage.
The innate immunity on diet.
Can we neuter the bonkers neutrophils?

Two and a half men poster by Sergio Kato | CC BY 2.0.
Talking of innate immune cells, remember I told you about neutrophils in the last blog. The immune cells that are first to arrive at the site of injury or infection. However, when they overstay their welcome they tend to cause tissue damage aggravating the inflammation further. We also learnt that during ageing the neutrophils tend to have defective migration both into and out of the tissue.
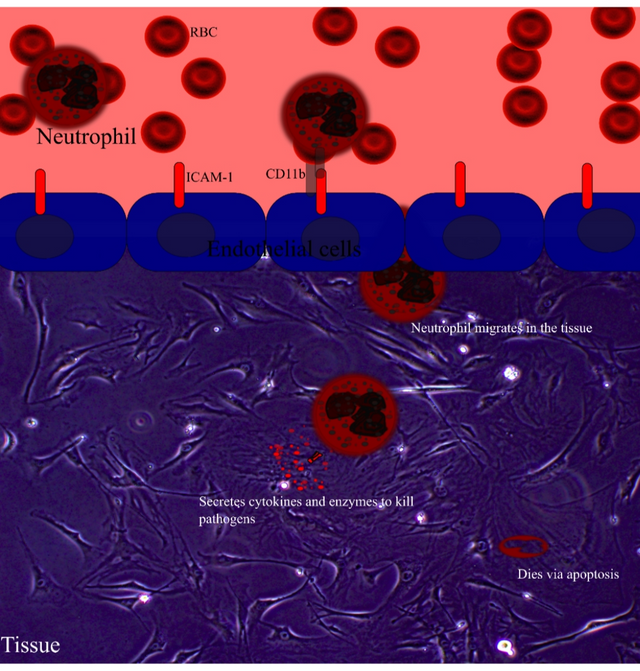
Illustration by @scienceblocks in inkspace.
Tissue image is image of fibroblasts on a dish taken by me. RBC image is by sbtlneet | pixabay
Their adhesion receptor profile also changes. It appears that Beta 2 integrin in aged neutrophils binds more strongly to ICAM-1. This may make them adhere strongly to the tissue they invade into and may also prolong their survival. It comes as no surprise their death via apoptosis is not like normal young cells making them overstay their welcome. Moreover, neutrophils are produced in bone marrow, and then after some rounds in circulation, they return to bone marrow and die. However, that going to bone marrow and dying part doesn't work out in aged neutrophils. Well, if they don't go and die they have to go somewhere. Maybe, that's one of the reasons why there is unwanted low-grade inflammation in aged tissues. (Qian et al., 2014, Kolaczkowska, 2016). We also saw that their profile changes to a more damaging kind.
Fasting, on the other hand, has been shown to be beneficial in reducing inflammation in diseases such as rheumatoid arthritis. It is likely that at least part of this effect is mediated by targeting the neutrophil function - such as secretion of inflammatory cytokines. For instance, Hafström, in 1988, showed that fasting reduces leukotriene B4 production in neutrophils - a compound known to promote neutrophil migration and aggregation. If you think about it, one of the early pathway discovered to be regulated by caloric restriction is insulin-like growth factor/PI3K pathway. The caloric restriction seems to inhibit the PI3K/AKT pathway (Mercken et al., 2013). In older adults neutrophil shows high consecutive activation of PI3K in neutrophils (Naccache et al., 2014). Inhibiting PI3K using a pharmacological inhibitor for 2 isoforms of PI3K improves neutrophil migration accuracy in old neutrophils (Sapey et al., 2014). On the other hand, the aged neutrophils seem to employ p38 MAPK and TLR4 pathway to create their altered adhesion molecule and cytokine profile. And inhibition of these pathways seem to improve neutrophil function (Uhl et al., 2016).
In fact, recently Wu et al., showed that dietary restriction mediates an effect on innate immunity in C elegans via the p38 pathway. Too much of p38 activation caused hyperresponsive innate immune system, and dietary restriction got things to normal. However, when p38 was completely inhibited the lifespan was not extended despite caloric restriction. Indicating that the pathway needs to work at some optimal level for the innate immune system to behave. It would be interesting to a drug targeting p38 or PI3K specifically in immune cells can serve as the elixir of life or not?
How to get senile Macrophages back on track?
.png)
Image by A. Rad, Mikael Häggström, Spacebirdy, RexxS, domdomegg | CC BY-SA 4.0.
However, neutrophils are not the only cell of the innate immune system who lose their shit in old age. Macrophage, the next important innate immune cell come at the site of injury or infection also show increased cytokine production, esp TNFa and IL6. If you are wondering then TNFa is the same molecule against which biologicals are designed in treatment of autoimmune diseases. They too have defective chemotaxis and reduced phagocytotic activity. In a nutshell, they become this cynical person who hurt you by words but does little to tackle real issues. However, the caloric restriction comes to rescue once again.
For instance, in 2018, Trott et al., showed that macrophage infiltration into walls of the artery, along with senescent B and T cells increase with ageing. Given the macrophages forms the foam cells along with all the fat they eat in walls of arteries to cause arteriosclerosis; their accumulation may explain why the risk for this disease increase with ageing. But here is the cool part caloric restriction, as well as regular exercise, seem to fix the issue.
Moreover, as we age, the macrophages seem to be polarised to a M2 macrophage subtype (Lavie and Weinreb, 1996). M2 macrophages are more tumour friendly than M1 macrophages. They are part of the problem where the probability of getting cancer increases with ageing. However, once again it seems that caloric restriction can switch this polarization back to M1 type macrophages (Simone et al., 2018).
One of the mechanisms implicated in causing the aged phenotype of macrophage is a reduction in autophagy - a process in which cell it's its own component and renew them (Stranks et al., 2015). Since autophagy is induced by calorie restriction and fasting, it may be one of the mechanisms by which calorie restriction acts on macrophages. The macrophage quality maintenance by autophagy hence seems to another potential target for the elixir of life.
When the assassins get dementia, should we pay them less in terms of energy?
.jpeg)
Image by Simon Caulton | CC BY-SA 3.0
Now macrophage and neutrophils are of course the innate immune cells that first come to mind. However, that’s not where the story ends. Another important cell type which goes around killing virally infected cells and is an efficient killer of the cancer cell is the Natural killer (NK) cell. The name says it all – its an assassin of cells it doesn’t approve of. That includes all the senescent cells, as well. Funny thing though it seems that natural killer cell number seems to go up in ageing as per some studies (Grounder et al., 2018, Duggal, 2018). However, with ageing their production and proliferation rate drops. This may mean that old long-lived NK cells accumulate with age. Moreover, the NK cell in old people are more of a mature kind (Hazeldine and Lord, 2013).
Also, despite the increase in number there seem to be an increase in the risk of viral infections, cancer and amount of senescent cells in body as we age. Looks like someone is enjoying the party without doing their job. Anyway, one is the proposed explanation in this regard is subtype of natural killer cells that dominate with ageing. There are two known subtypes, known as CD56bright and CD56dim (I can explain you in comments as to why they are bright or dim). They have different binding and homing abilities. Also, the dim guys are more cytotoxic to other cells than the bright ones. The CD56dim kind seems to increase with age. Nevertheless, it appears that their ability to kill the cells by secreting certain enzymes do reduce with age (Hazeldine and Lord, 2013). And as if the changes in their homing and cytotoxicity is not enough, their migration also becomes defective in old people. In a nutshell, they might have difficulty in reaching the target tissues, and if they reach they don't do their job wholeheartedly. No wonder the senescent cells accumulate in the body.
Caloric restriction in mice seem to get rid of mature NK cells and increase the accumulation of immature young NK cells (White et al., 2016). In mice, the CD127+ NK has the same properties as CD56bright NK cells in humans. Clinthrone et al., in 2013, their numbers seem to higher in mice on calorie-restricted diet plus they produced more cytokines. Moreover, their cytotoxicity towards cancer cells seems to improve in these dietary restricted mice. But, the authors also show that their ability to respond to viral infections is however diminished.
The mechanism by which it occurs is not well understood. Also, while it is good to know that they calorie-restricted NK cells become effective in responding to cancer cells, the loss of response to viral infection is a matter of concern and needs further study. If we can understand this mechanism better maybe we can come up with a drug that improves NK cell function without making them less effective towards viral infections.
Another mechanism by which caloric restriction might act on NK cells is indirectly via a hormone called leptin. Yeah, the same hormone responsible for hunger. Since leptin regulates NK cells and dietary restriction diminish leptin levels it may be one of the way through which at least NK cell numbers are affected via diet (Duggal, 2018).
Elixirs that may target inflammageing.
Ok, I had some more to talk about inflammatory cytokines and effect of calorie restriction on non-immune cells in countering inflammageing. I also did not talk much about B cells and dendritic cells. However, for today I will press the breaks here and summarize what we discussed. That part I can, in fact, cover in future episodes. However, before we close this a few remarks on some potential Elixirs of life.
What we saw mostly is that calorie restriction reduces inflammation and can slow down inflammageing. It acts by slowing the senescence of immune cells in both adaptive and innate arms of immunity. Though we also became aware of some of the side effects of calorie-restricted diets. Such as further reduced production of T cells and a possible lack of resistance to pathogens. Though this needs further exploration, it's better to be aware of this if you are cutting down your calories. Moreover, we did witness some new targets for the elixir of life - autophagy, PI3K and p38.
Go! Eat yoursef.
In this regard, genetically, promoting autophagy in mice increases their lifespan (Farnandez et al., 2018). Since, it's possible to cause mutation in some molecules, induce autophagy and increase lifespan, it would be interesting to see a drug that can target these molecules pharmacologically.
Let's target PI3K
As far as PI3K is concerned, then studies in fruitfly and nematodes show that pharmacological inhibition of PI3K extends lifespan (Moskalev et al., 2008, Baharill et al., 2013). It's partial inhibition genetically or via drug esp of PI3Kalpha, reduces obesity in mice and increases lifespan (Lopez-Guadamillas et al., 2016). However, one needs to be careful because some reports of trials of PI3K inhibitor for cancer implicates its insulin resistance. And, it may even hamper the function of other immune cells such as dendritic cells(Duggal, 2018). So much more enquiry in this would be required. Also, it would be interesting to see if the specific knockout of PI3K in specific immune cells can recapitulate the lifespan extension.
Ok, what about p38 MAPK
We already saw above that caloric restriction extends the lifespan of worms by downregulation of p38 in innate immune cells. Moreover, P38 inhibition has a protective effect on hematopoietic stem cells (Ito et al., 2006). Moreover, also increases the proliferative capacity of T cells (Duggal, 2018). It remains to be seen how it acts out in lifespan extension in mammals. Nevertheless, p38 is an important gene and inhibiting it in a systemic manner may cause a lot of other unwanted things. Hence, we would need a better understanding of where it should be inhibited and where not to touch it. That way, we can come up with a better strategy.
Other caloric restriction memetics
Oh! those diabetes pills!
Also, I should mention that some of the calorie restriction mimetic compounds, that we saw in previous episodes also act as an anti-inflammatory. For instance, metformin, the drug that is taken by diabetic patients and those trying to lose weight, have also been shown to increase lifespan in animal models. We have previously seen that this drug is an AMPK activator and acts via improving mitochondrial function. It has also been implicated in directly targeting the inflammation in the body, perhaps by AMPK inactivating NFkB (Saisho and Yoshifumi, 2015). It has also been shown that in patients suffering from arthritis, metformin reduces the inflammation-causing Th17 cells and upregulate inflammation suppressing T regulatory cells (Son et al., 2014). Also, AMPK activation seems to improve neutrophil function (Park et al., 2013). The tumours treated with metformin seem to have a reduction in M2 macrophages and an increase in M1 macrophages (Chiang et al., 2017). Furthermore, it also improves thymus function in diabetic patients (Dworacki et al., 2015). However, it remains to be seen how it acts out in the context of immunosenescence and ageing.
Rap the rapamycin?
Since AMPK also inactivates mTOR, we can ask if some of the immunomodulatory effects of metformin are also mediated via the mTOR pathway. Or you may ask directly that what if we inhibited mTOR via rapamycin? Well, rapamycin has been used as an anti-inflammatory drug (Duggal, 2018). Moreover, it seems to improve the functioning of T cells, dendritic cells and even hematopoietic stem cells (HSCs). At the beginning of the article I told you that caloric restriction while kept HSC in the quiescent state it did not improve its defective lymphocyte generation (Chen et al., 2009). Though rapamycin due to its side effects might not be the perfect elixir of life, it does gives us hint to what we may need to target in addition to caloric restriction.
The grapes vine and the red wine
You may also recall that compound I talked about in the previous posts. Yes, the one found in grapes and red wine - resveratrol. Guess what it also has an effect on inflammation. It affects neutrophils, T cells and even improves NK cell cytotoxicity (Duggal, 2018). However, more detailed studies on this would be required to understand its mechanism of action on different immune cells.
Final remarks
In a nutshell, not only caloric restriction seems to slow down immune ageing but so does the exercise and compounds that mimic caloric restriction. However, we still haven't figured out something to stop it altogether. But every year with all the research we are getting closer in making the picture clear. In any case, we will continue this quest in the next episode and try to get as close to the roots of ageing as we can.
About steemstem
But, before I go I would like to mention about the steemstem platform. Well, if you love reading and writing interesting science articles @steemstem is a community on steem that support authors and content creators in STEM field. If you wish to support steemstem do see the links below.
You can vote for steemstem witness here -Quick link for voting for the SteemSTEM Witness(@stem.witness)
Quick delegation links for @steemstem
50SP | 100SP | 500SP | 1000SP | 5000SP | 10000SP).
Delegating to @steemstem gives ROI of 65% of the curation rewards.
Also, if you have any questions regarding steemstem, do join the steemstem discord server.
You can DM me on discord, I have the same handle - @scienceblocks. Also if you are not a steem user, and reading this blog inspired you to start your science blog, find me on discord and let me know about you. I can try and help you navigate your way through steem.
References
The quest for elixir of life - understanding caloric restriction (episode 1 - DNA damage and repair).
The bugs and debugging of DNA - a quick refresher for DNA damage repair
The quest for elixir of life - understanding caloric restriction (episode 2 - mitochondria)
White et al., 2017. Calorie Restriction Attenuates Terminal Differentiation of Immune Cells
Kolaczkowska, 2016. The older the faster: aged neutrophils in inflammation
Paul H. Naccache and Julie S. Lefebvre, 2014. A straight neutrophil path to healthy aging?
Stranks et al., 2015. Autophagy Controls Acquisition of Aging Features in Macrophages
Saisho and Yoshifumi, 2015. Metformin and Inflammation: Its Potential Beyond Glucose-lowering Effect
Lopez-Guadamillas et al., 2016. PI3Ka inhibition reduces obesity in mice.

You are amazing. It's humbling to read this, and yet there is not one drop of arrogance in this blog. Wow. A research scientist will eat this stuff up. But better than that, I enjoyed it. I got a lot out of it and I've never been anywhere near a research lab. Although I did volunteer for the biology lab in high school :)
Bottom line, as you explain it, there is no elixir. Moderation once again seems to be the best path. Perhaps a little wine and very modest dietary restriction --- until we learn more.
Thank you so much for preparing such a comprehensive overview for us. I will refer to this in the future as I seek to verify less well-documented information from other sources.
Have a great day, @scienceblocks. It makes me smile to know you write for SteemSTEM, for all of us.
Resteeming and sharing on Twitter.
Not sure how to thank you for this amazing feedback. But wow, thanks. This really wi keep me motivated. Also glad that I could get the information across.
Posted using Partiko Android
Every now and then you come across someone who reminds you how you should behave, what's important in work. That's what the quality of your work did for me yesterday. Thanks.
I am happy to have found this post tonight. I will probably have a look to the first three episodes during the next days. I unfortunately miss them…
I learned a few things around here, like those benefits about caloric restrictions. I know that having a proper diet contributes to a healthier life (when compared with calorimetric excesses), but I didn’t know it was affecting chronic diseases, and could affect how our immune system ages. I am looking forward to read the rest. I assume there is no unique combination of ways of living that stops ageing (otherwise we would know it, I guess), but I am wondering what else could impact it. I will probably have to wait a bit for an answer, won't I?
I think CR is somewhat most consistent thing found to work in many animal models. Maybe in future, we will also figure out that more than calories it has been the synergistic effect of reducing certain molecules in the diet. It's interesting to use CR as a proxy, for now, to figure out the molecular pathways. If we know the pathways we can have some guess on what genes might be involved, what are the gene-environmental interactions apart from a diet that slows or accelerates ageing. If I compare the field of ageing with particle physics we have just shot electrons on a gold foil to guess the structure of atoms. It's a long way until we get to the standard model.
Wow @scienceblocks, what a wonderful summary about elderly immune cells. Never encountered something like this before, respect. And yes, I 100% agree with you.
Eat less, stay alive!
Best regards
Chapper
Oh: Resteem ;-)
I've encountered stuff like this before...on your blog. No disrespect for @scienceblocks. My jaw dropped as I read through this blog. Amazing. Makes me hesitate to write. What a standard @scienblock sets. But I also kept thinking of the blogs you wrote about mitochondria. Pretty amazing. You two should talk :)) Sets the standard for SteemSTEM professionalism really, really high. Plus, @scienceblocks does funny drawings also :))
I completely agree with you @agmoore2. Chapper is amazing. I remember the first time I read his posts, I sent him the discord link to steemstem immediately.
Thanks for knowledgeable post.
This post has been voted on by the SteemSTEM curation team and voting trail. It is elligible for support from @curie and @utopian-io.
If you appreciate the work we are doing, then consider supporting our witness stem.witness. Additional witness support to the curie witness and utopian-io witness would be appreciated as well.
For additional information please join us on the SteemSTEM discord and to get to know the rest of the community!
Thanks for having added @steemstem as a beneficiary to your post. This granted you a stronger support from SteemSTEM.
Thanks for having used the steemstem.io app. You got a stronger support!
Hi @scienceblocks!
Your post was upvoted by @steem-ua, new Steem dApp, using UserAuthority for algorithmic post curation!
Your UA account score is currently 3.581 which ranks you at #6312 across all Steem accounts.
Your rank has improved 240 places in the last three days (old rank 6552).
In our last Algorithmic Curation Round, consisting of 366 contributions, your post is ranked at #167.
Evaluation of your UA score:
Feel free to join our @steem-ua Discord server