The Wonders of the Electro-Magnetic Spectrum - Light Interaction - Why You See the Colours You Do!
We will look at the EM-spectrum, pointing out interesting facts.
Lights fundamental interaction with matter, atomic transitions, energy levels, photo-electric effect.
Do you know why you see the colours you do? We will discuss what happens at the atomic level.
Image Credit:-freegreatpicture
Physics.Benjamin
Introduction
In today's article I am going to talk about the electromagnetic spectrum and the different types of light. Light has a fundamental relationship with matter and atoms, the energy levels in atoms can be excited by particles of light. This interaction allows for identification of atoms. Finally we will look at the process behind seeing light, why does that green apple look green? Hopefully with the information presented in this article you'll have a better understanding of different types of light and how it interact at the atomic scale.
Electromagnetic Spectrum
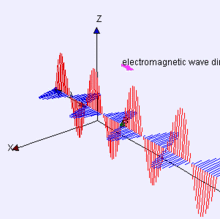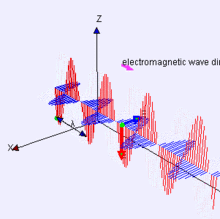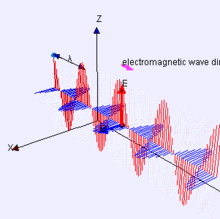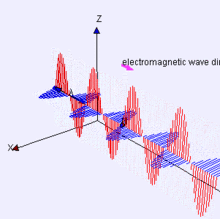
Image Credit:-wikipedia
Representation of EM-wave, see how the electric and magnetic parts move like waves, are right angle to each other.
The nature of light is a funny thing, light speed defines an absolute limit to the speed something can travel. No particle of matter can ever reach light speed or it will break laws of physics, it will have infinite energy and mass, which can't be possible with the model we observe. We talk about light as waves, indeed it shows all the characteristics of waves, such as diffraction and interference for example. However we also see light behave as little packets of energy which we call photons, we say that are quantised. This is an important fact to remember.
So light behaves as waves with frequency(number of waves per second) and a wavelength(the length between two waves), these are always proportional to each other because they travel at the speed of light.
The higher frequency of the wave the more energy it possesses, in the photon, and the wavelength will be shorter. High frequency = High energy = Short wavelength.
The lower frequency of the wave the less energy it possesses, in the photon, and the wavelength will be longer. Low frequency = Low energy = Long wavelength.
Due to the nature of light, there is an infinite number of possible frequencies and exact energies a photon may have. This is a profound feature of light. Any possible value of energy is possible with light.

Image Credit:-wikimedia
Now you should be familiar with different types of light, radio waves are indeed light, and so are x-rays. Radio waves are low energy but can have wavelengths up to Kilometres long in some cases. X-rays are short wavelength sometimes 10 picometers (that's 10 with to 12 decimal places), these are highly energetic and ionise atoms (an electron is removed from the atom).
Gamma rays
Gamma rays are extremely energetic and there come from very energetic sources. We observe them in space coming from explosions in galaxies and also during a nuclear reaction when gamma radiation is produced. They are very dangerous as they have enough energy to break the chemical bonds in our atoms. So they can have adverse effects on our DNA and overall health.
Quick note alpha, beta and gamma radiation are completely different, go check it out
X-rays
The next energetic form of light is X-rays, you'll be familiar with this is you've ever broken a bone and had to go to hospital for an X-ray. An x-ray is quickly generated with something called a cathode, and the photons travel through the body some of them are absorbed by the atoms in your body. The move dense the tissue such as bone, the more atoms is contains and will absorb more.The x-ray you see is a negative image, the bone is very clear in the image, but is has absorbed the most light, the darker areas were exposed to more x-rays. They are highly ionising, meaning the light can easily remove electrons from the atom. Exposure to these can cause serious effects. We see very hot plasma in space called nebulae, some are left over from a supernovae explosion, and they emits a lot if x-ray radiation.
Ultraviolet
Ultra-violet is next, this is the light that makes white clothes glow blue at night clubs. This type of light is kind of on the border of being harmful, and actually visible. It doesn't ionise but ejecting and electron but it does have energy to change the energy levels of atoms and cause chemical reactions. The higher energetic end of the spectrum is harmful for the reason I just explained, this is the type of UV light you must protect yourself with on a sunny day. However some frequencies of UV light are beneficial for vitamin D production. As the frequency of the UV light decreases it becomes less harmful, and the bluest end of the visible light is nearly UV light. Bees are believed to see UV light, and flowers have leafs that are constructed to be more beautiful in the UV spectrum.
Visible light
Visible light is exactly that, it's the light our human eyes can see from blue which is high energy to red which is lower energy The light range we can see have wavelengths 400-700 nano meters, nano is to 9 decimal places. The put into scale how much of the spectrum of light we see, consider that radio waves can have wavelengths of kilo meters. We literally see a tiny portion of the EM-spectrum, which we see as the colours of the rainbow. The visible light emitted from the sun is mostly is the visible spectrum, and it emits a higher percentage of yellow light, hence the yellow appearance. I'll discuss a little bit more visible light in the last section.
Infrared
This is the light we mostly associate with heat, infrared cameras detect the heat emitted by an object. It has the name infra-red because it extends into the reddest part of the visible spectrum, but is invisible to the human eye. In fact it was William Herschel a great astronomer who discovered his. He was splitting the visible light from the sun through a prism and using a thermometer to detect its heat, he found that an invisible light was causing the temperature to rise the most. The reason it heat is because its frequency regularly causes the molecules to vibrate, which translates to kinetic energy of the molecule, which is just how "hot" it is. Something is hot because it's moving or vibrating fast, at the atomic level. The body heat you generate through physiological processes causes the molecules in your body to vibrate, and these vibrations basically cause you to emit infrared light as a result. This light you have emitted from the day you were born, this light has travelled through the solar system and beyond. If you are 25 years old, then you have a sphere around you that's 25 light years big that contains the infrared light you have emitted. It is was possible to distinguish between light photons you could detect your photons in a huge sphere lare than the solar system, that has reach some of our closest stars.
Image credit:-wikimedia
Micro waves and Radio waves
These exist at the lower end of the spectrum in terms of energy. How ever let me explain the microwave quickly, the more energetic microwaves are the right frequency to resonant a hydrogen-bond. When a microwave cooker heats something up it emits microwaves that vibrates the hydrogen bonds in the food, these vibration basically causes it to heat, movement is heat at the atomic level. Mostly these are used in communications from radio transmitters to your wifi and satellite communication. Microwaves have a range of wavelength 1mm-1m and radio waves can be up to kilometres long and even longer in theory. There is a limit on the how short a gamma ray wavelength can be, bu radio waves can theoretically have lengths comparable to galaxies.
Interaction of Light and Matter - Energy Levels and Transitions
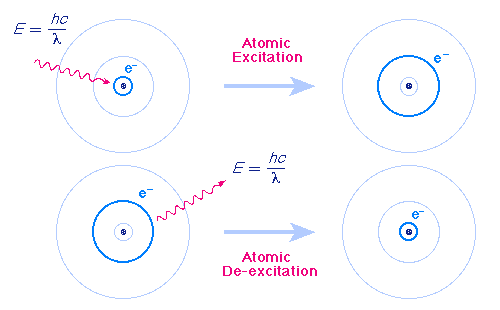
Image Credit:-Source
Atoms and molecules have energy levels associated to the electrons. Atoms can be excited from their ground state so that electrons orbit the atoms with high energies. These energies of the electrons are discrete, they take specific values depending on the atom or molecule. There is no intermediate zone between electron energy levels. Due to this fact there is an exact difference in energy between two two levels. For example one energy level could have 1 electron volts (a unit of energy) and the higher level 3 electron volts, so the difference would be 2 eV. If light hits the atom with the exact energy of 2 eV then the electron will be excited to the higher energy level. Now it is excited, it naturally wants to relax, and by doing so decays back to the lower energy level emitting a photon of 2 eV, exactly the same that excited it. When light passes through a gas, if some of the light matches the transition energy of the gas atoms, it will be absorbed, if we look at the spectrum of light we see that some of it is missing due to being absorbed, this is called absorption spectra. Every atom and molecule has its own finger print that is it's absorption spectra.
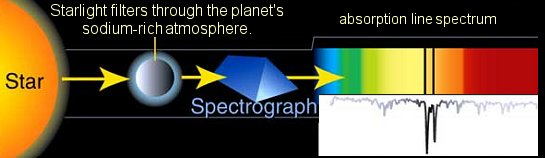
Image credit:-wikimedia
Notice in the image that the sodium in the planets atmosphere is absorbed, so some of the yellow light is mission.
Equivalently, there is an emission spectra, atoms will emit photons corresponding to the energy difference between two levels. So for sodium you would see the exact opposite as above, you'd see two yellow lines and only that, these would identify the presence of sodium.

Image credit:-wikimedia
Emission spectra of hydrogen, notice that only the colour corresponding to energy level transitions are visible.
I think this theory is very important to understand in all aspects of science. It can be used for speeding up chemical reactions and is used in different areas of biology. Light can change
Why You See Colours!
Image credit:-source
Notice the other colours of light is absorbed but the green light i reflected. You would see green light as a result.
Have you ever wondered why you see colours of light. Well sir Isaac Newton discovered that materials absorb most of the white light and then reflects a small range of visible light. For example a green tennis ball absorbs all the colours and reflects the green color. If you have ever seen fluorescent light, this is light of a certain colour being emitted from the atoms, but it has a small range of frequencies. When you see the green tennis ball, it is a mix of green frequencies that you see, the ball reflects a range of green instead of a single frequency, its called the bandwidth.
The white light hits an object, it absorbs most of the light, then reflects a certain colour over a range.
JN Tinsley et al. Nature Comms 7, 12172 (2016) actually suggests that a human can detect a single photon of light, in the visible range. There is argument over how many photons does it take to cause a visual excitement, but studies suggest there is a mechanism to amplify the signal we receive from detecting the light.
Conclusion
There exists a wide range of different types of light. Higher energy light has shorter wavelength and higher frequency, for example gamma waves. At the lower energy of the spectrum is radio waves that can have very long wavelengths. They can exist an infinite number of different energy light in theory, you rarely find nature being exact with us. The electron energy levels of an atom can be excited by light that matches the right energy, it then relaxes emitting the same type of light it just absorbed. And if anyone ever asks you why is the apple green, then you know it's because it absorbs everything else and reflects only the green light.
I hope you found this article interesting. The study of electromagnetism really is a world of it's own, and it takes the greatest mind to fully grasp it's true nature. Until next time.....
Physics.Benjamin
If you liked this post feel free to UPVOTE, FOLLOW, and RESTEEM.
References:-
Electromagnetic Spectrum
Gamma rays
X-rays
Ultraviolet
Visible light
Infrared
Microwaves
Radio waves
Emission spectrum
Absorbtion spectrum
All images are Creative Commons or public domain, no copyright infringements have occurred.



Congratulations! Your post has been selected as a daily Steemit truffle! It is listed on rank 17 of all contributions awarded today. You can find the TOP DAILY TRUFFLE PICKS HERE.
I upvoted your contribution because to my mind your post is at least 17 SBD worth and should receive 78 votes. It's now up to the lovely Steemit community to make this come true.
I am
TrufflePig, an Artificial Intelligence Bot that helps minnows and content curators using Machine Learning. If you are curious how I select content, you can find an explanation here!Have a nice day and sincerely yours,

TrufflePigThank you for the recognition.